Highly differentiated human airway epithelial cells: a model to study host cell-parasite interactions in pertussis
- PMID: 26492208
- PMCID: PMC5278880
- DOI: 10.3109/23744235.2015.1100323
Highly differentiated human airway epithelial cells: a model to study host cell-parasite interactions in pertussis
Abstract
Background: Bordetella pertussis colonizes the human respiratory mucosa. Most studies on B. pertussis adherence have relied on cultured mammalian cells that lack key features present in differentiated human airway cells or on animal models that are not natural hosts of B. pertussis. The objectives of this work were to evaluate B. pertussis infection in highly differentiated human airway cells in vitro and to show the role of B. pertussis fimbriae in cell adherence.
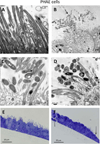
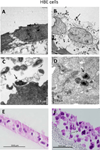
Methods: Primary human airway epithelial (PHAE) cells from human bronchi and a human bronchial epithelial (HBE) cell line were grown in vitro under air-liquid interface conditions.
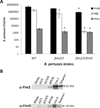
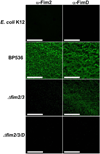
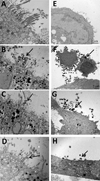
Results: PHAE and HBE cells infected with B. pertussis wild-type strain revealed bacterial adherence to the apical surface of cells, bacteria-induced cytoskeleton changes, and cell detachment. Mutations in the major fimbrial subunits Fim2/3 or in the minor fimbrial adhesin subunit FimD affected B. pertussis adherence to predominantly HBE cells. This cell model recapitulates the morphologic features of the human airway infected by B. pertussis and confirms the role of fimbriae in B. pertussis adherence. Furthermore, HBE cells show that fimbrial subunits, and specifically FimD adhesin, are critical in B. pertussis adherence to airway cells.
Conclusions: The relevance of this model to study host-parasite interaction in pertussis lies in the striking physiologic and morphologic similarity between the PHAE and HBE cells and the human airway ciliated and goblet cells in vivo. These cells can proliferate in vitro, differentiate, and express the same genetic profile as human respiratory cells in vivo.
Keywords: Bordetella pertussis; adherence; fimbriae major subunit Fim2 or Fim3; minor subunit FimD.
Figures

References
-
- Burns DL, Meade BD, Messionnier NE. Pertussis resurgence: perspectives from the Working Group Meeting on pertussis on the causes, possible paths forward, and gaps in our knowledge. J Infect Dis. 2014;209(Suppl 1):S32–S35. - PubMed
-
- Crowcroft NS, Stein C, Duclos P, Birmingham M. How best to estimate the global burden of pertussis? Lancet Infect Dis. 2003;3(7):413–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P60 DK020593/DK/NIDDK NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- R01 AI101055/AI/NIAID NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- IK2 BX001701/BX/BLRD VA/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
