Functional Impairment of Myeloid Dendritic Cells during Advanced Stage of HIV-1 Infection: Role of Factors Regulating Cytokine Signaling
- PMID: 26492336
- PMCID: PMC4619614
- DOI: 10.1371/journal.pone.0140852
Functional Impairment of Myeloid Dendritic Cells during Advanced Stage of HIV-1 Infection: Role of Factors Regulating Cytokine Signaling
Abstract
Introduction: Severely immunocompromised state during advanced stage of HIV-1 infection has been linked to functionally defective antigen presentation by dendritic cells (DCs). The molecular mechanisms behind DC impairment are still obscure. We investigated changes in DC function and association of key regulators of cytokine signaling during different stages of HIV-1 infection and following antiretroviral therapy (ART).
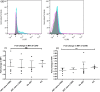
Methods: Phenotypic and functional characteristics of circulating myeloid DCs (mDCs) in 56 ART-naive patients (23 in early and 33 in advanced stage of disease), 36 on ART and 24 healthy controls were evaluated. Sixteen patients were studied longitudinally prior-to and 6 months after the start of ART. For functional studies, monocyte-derived DCs (Mo-DCs) were evaluated for endocytosis, allo-stimulation and cytokine secretion. The expression of suppressor of cytokine signaling (SOCS)-1 and other regulators of cytokine signaling was evaluated by real-time RT-PCR.
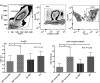
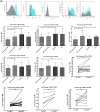
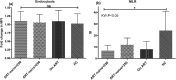
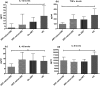
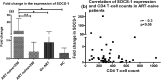
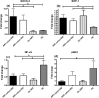
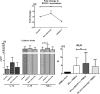
Results: The ability to respond to an antigenic stimulation was severely impaired in patients in advanced HIV-1 disease which showed partial recovery in the treated group. Mo-DCs from patients with advanced HIV-disease remained immature with low allo-stimulation and reduced cytokine secretion even after TLR-4 mediated stimulation ex-vivo. The cells had an increased expression of negative regulatory factors like SOCS-1, SOCS-3, SH2-containing phosphatase (SHP)-1 and a reduced expression of positive regulators like Janus kinase (JAK)2 and Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)1. A functional recovery after siRNA mediated silencing of SOCS-1 in these mo-DCs confirms the role of negative regulatory factors in functional impairment of these cells.
Conclusions: Functionally defective DCs in advanced stage of HIV-1 infection seems to be due to imbalanced state of negative and positive regulatory gene expression. Whether this is a cause or effect of increased viral replication at this stage of disease, needs further investigation. The information may be useful in design of novel therapeutic targets for better management of disease.
Conflict of interest statement
Figures
Similar articles
-
Increased expression of negative regulators of cytokine signaling during chronic HIV disease cause functionally exhausted state of dendritic cells.Cytokine. 2017 Mar;91:118-123. doi: 10.1016/j.cyto.2016.08.010. Epub 2017 Jan 3. Cytokine. 2017. PMID: 28056393
-
Enhanced induction of HIV-specific cytotoxic T lymphocytes by dendritic cell-targeted delivery of SOCS-1 siRNA.Mol Ther. 2010 Nov;18(11):2028-37. doi: 10.1038/mt.2010.148. Epub 2010 Jul 20. Mol Ther. 2010. PMID: 20648001 Free PMC article.
-
IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells.J Leukoc Biol. 2006 Jun;79(6):1279-85. doi: 10.1189/jlb.0905503. Epub 2006 Mar 21. J Leukoc Biol. 2006. PMID: 16551679
-
Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins.Immunobiology. 2008;213(3-4):225-35. doi: 10.1016/j.imbio.2007.10.008. Epub 2007 Nov 28. Immunobiology. 2008. PMID: 18406369 Review.
-
[Regulation of cytokine and toll-like receptor signaling by SOCS family genes].Nihon Rinsho. 2004 Dec;62(12):2189-96. Nihon Rinsho. 2004. PMID: 15597784 Review. Japanese.
Cited by
-
Antiretroviral therapy partially improves the abnormalities of dendritic cells and lymphoid and myeloid regulatory populations in recently infected HIV patients.Sci Rep. 2019 Aug 12;9(1):11654. doi: 10.1038/s41598-019-48185-2. Sci Rep. 2019. PMID: 31406185 Free PMC article.
-
Dendritic Cells in HIV/SIV Prophylactic and Therapeutic Vaccination.Viruses. 2019 Dec 24;12(1):24. doi: 10.3390/v12010024. Viruses. 2019. PMID: 31878130 Free PMC article. Review.
-
Isolation, Transfection, and Culture of Primary Human Monocytes.J Vis Exp. 2019 Dec 16;(154):10.3791/59967. doi: 10.3791/59967. J Vis Exp. 2019. PMID: 31885371 Free PMC article.
References
-
- Levy JA, Scott I, Mackewicz C. Protection from HIV/AIDS: the importance of innate immunity. Clin Immunol. 2003; 108: 167–174. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998; 392: 245–252. - PubMed
-
- Rowland-Jones SL. Timeline: AIDS pathogenesis: what have two decades of HIV research taught us? Nat Rev Immunol. 2003; 3: 343–348. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

