Embryonic Poly(A)-Binding Protein (EPAB) Is Required for Granulosa Cell EGF Signaling and Cumulus Expansion in Female Mice
- PMID: 26492470
- PMCID: PMC4701890
- DOI: 10.1210/en.2015-1135
Embryonic Poly(A)-Binding Protein (EPAB) Is Required for Granulosa Cell EGF Signaling and Cumulus Expansion in Female Mice
Abstract
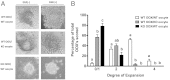
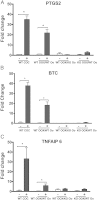
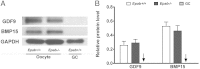
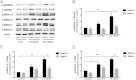
Embryonic poly(A)-binding protein (EPAB) is the predominant poly(A)-binding protein in Xenopus, mouse, and human oocytes and early embryos before zygotic genome activation. EPAB is required for translational activation of maternally stored mRNAs in the oocyte and Epab(-/-) female mice are infertile due to impaired oocyte maturation, cumulus expansion, and ovulation. The aim of this study was to characterize the mechanism of follicular somatic cell dysfunction in Epab(-/-) mice. Using a coculture system of oocytectomized cumulus oophorus complexes (OOXs) with denuded oocytes, we found that when wild-type OOXs were cocultured with Epab(-/-) oocytes, or when Epab(-/-) OOXs were cocultured with WT oocytes, cumulus expansion failed to occur in response to epidermal growth factor (EGF). This finding suggests that oocytes and cumulus cells (CCs) from Epab(-/-) mice fail to send and receive the necessary signals required for cumulus expansion. The abnormalities in Epab(-/-) CCs are not due to lower expression of the oocyte-derived factors growth differentiation factor 9 or bone morphogenetic protein 15, because Epab(-/-) oocytes express these proteins at comparable levels with WT. Epab(-/-) granulosa cells (GCs) exhibit decreased levels of phosphorylated MEK1/2, ERK1/2, and p90 ribosomal S6 kinase in response to lutenizing hormone and EGF treatment, as well as decreased phosphorylation of the EGF receptor. In conclusion, EPAB, which is oocyte specific, is required for the ability of CCs and GCs to become responsive to LH and EGF signaling. These results emphasize the importance of oocyte-somatic communication for GC and CC function.
Figures


Similar articles
-
Embryonic poly(A)-binding protein (EPAB) is required for oocyte maturation and female fertility in mice.Biochem J. 2012 Aug 15;446(1):47-58. doi: 10.1042/BJ20120467. Biochem J. 2012. PMID: 22621333 Free PMC article.
-
Growth differentiation factor 9 signaling requires ERK1/2 activity in mouse granulosa and cumulus cells.J Cell Sci. 2010 Sep 15;123(Pt 18):3166-76. doi: 10.1242/jcs.063834. Epub 2010 Aug 24. J Cell Sci. 2010. PMID: 20736313
-
Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling.Mol Endocrinol. 2010 Jun;24(6):1230-9. doi: 10.1210/me.2009-0497. Epub 2010 Apr 9. Mol Endocrinol. 2010. PMID: 20382892 Free PMC article.
-
The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process.J Reprod Dev. 2012;58(5):510-4. doi: 10.1262/jrd.2012-056. J Reprod Dev. 2012. PMID: 23124701 Review.
-
The epidermal growth factor network: role in oocyte growth, maturation and developmental competence.Hum Reprod Update. 2018 Jan 1;24(1):1-14. doi: 10.1093/humupd/dmx029. Hum Reprod Update. 2018. PMID: 29029246 Review.
Cited by
-
Translational activation of maternally derived mRNAs in oocytes and early embryos and the role of embryonic poly(A) binding protein (EPAB).Biol Reprod. 2019 May 1;100(5):1147-1157. doi: 10.1093/biolre/ioz034. Biol Reprod. 2019. PMID: 30806655 Free PMC article. Review.
-
Epidermal growth factor receptor signaling uncouples germ cells from the somatic follicular compartment at ovulation.Nat Commun. 2021 Mar 4;12(1):1438. doi: 10.1038/s41467-021-21644-z. Nat Commun. 2021. PMID: 33664246 Free PMC article.
-
Transcriptomic landscape of cumulus cells from patients <38 years old with a history of poor ovarian response (POR) treated with platelet-rich plasma (PRP).Aging (Albany NY). 2025 Feb 18;17(2):431-447. doi: 10.18632/aging.206202. Epub 2025 Feb 18. Aging (Albany NY). 2025. PMID: 39976580 Free PMC article.
-
Depletion of placental brain-derived neurotrophic factor (BDNF) is attributed to premature ovarian insufficiency (POI) in mice offspring.J Ovarian Res. 2024 Jul 9;17(1):141. doi: 10.1186/s13048-024-01467-4. J Ovarian Res. 2024. PMID: 38982490 Free PMC article.
-
Paxillin and embryonic PolyAdenylation Binding Protein (ePABP) engage to regulate androgen-dependent Xenopus laevis oocyte maturation - A model of kinase-dependent regulation of protein expression.Mol Cell Endocrinol. 2017 Jun 15;448:87-97. doi: 10.1016/j.mce.2017.03.028. Epub 2017 Mar 28. Mol Cell Endocrinol. 2017. PMID: 28359799 Free PMC article.
References
-
- Eppig JJ, Schultz RM, O'Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol. 1994;164:1–9. - PubMed
-
- Adashi EY. Endocrinology of the ovary. Hum Reprod. 1994;9:815–827. - PubMed
-
- Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. BioEssays. 1991;13:569–574. - PubMed
-
- Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

