TIM-3 Suppresses Anti-CD3/CD28-Induced TCR Activation and IL-2 Expression through the NFAT Signaling Pathway
- PMID: 26492563
- PMCID: PMC4619610
- DOI: 10.1371/journal.pone.0140694
TIM-3 Suppresses Anti-CD3/CD28-Induced TCR Activation and IL-2 Expression through the NFAT Signaling Pathway
Abstract
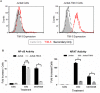
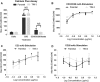
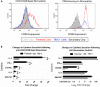
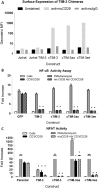
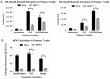
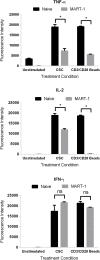
TIM-3 (T cell immunoglobulin and mucin-domain containing protein 3) is a member of the TIM family of proteins that is preferentially expressed on Th1 polarized CD4+ and CD8+ T cells. Recent studies indicate that TIM-3 serves as a negative regulator of T cell function (i.e. T cell dependent immune responses, proliferation, tolerance, and exhaustion). Despite having no recognizable inhibitory signaling motifs, the intracellular tail of TIM-3 is apparently indispensable for function. Specifically, the conserved residues Y265/Y272 and surrounding amino acids appear to be critical for function. Mechanistically, several studies suggest that TIM-3 can associate with interleukin inducible T cell kinase (ITK), the Src kinases Fyn and Lck, and the p85 phosphatidylinositol 3-kinase (PI3K) adaptor protein to positively or negatively regulate IL-2 production via NF-κB/NFAT signaling pathways. To begin to address this discrepancy, we examined the effect of TIM-3 in two model systems. First, we generated several Jurkat T cell lines stably expressing human TIM-3 or murine CD28-ECD/human TIM-3 intracellular tail chimeras and examined the effects that TIM-3 exerts on T cell Receptor (TCR)-mediated activation, cytokine secretion, promoter activity, and protein kinase association. In this model, our results demonstrate that TIM-3 inhibits several TCR-mediated phenotypes: i) NF-kB/NFAT activation, ii) CD69 expression, and iii) suppression of IL-2 secretion. To confirm our Jurkat cell observations we developed a primary human CD8+ cell system that expresses endogenous levels of TIM-3. Upon TCR ligation, we observed the loss of NFAT reporter activity and IL-2 secretion, and identified the association of Src kinase Lck, and PLC-γ with TIM-3. Taken together, our results support the conclusion that TIM-3 is a negative regulator of TCR-function by attenuating activation signals mediated by CD3/CD28 co-stimulation.
Conflict of interest statement
Figures

Similar articles
-
Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways.Mol Cell Biol. 2011 Oct;31(19):3963-74. doi: 10.1128/MCB.05297-11. Epub 2011 Aug 1. Mol Cell Biol. 2011. PMID: 21807895 Free PMC article.
-
Down-regulation of interleukin-2 production by CD4(+) T cells expressing TIM-3 through suppression of NFAT dephosphorylation and AP-1 transcription.Immunobiology. 2012 Oct;217(10):986-95. doi: 10.1016/j.imbio.2012.01.012. Epub 2012 Jan 16. Immunobiology. 2012. PMID: 22445722
-
Molecular pathway profiling of T lymphocyte signal transduction pathways; Th1 and Th2 genomic fingerprints are defined by TCR and CD28-mediated signaling.BMC Immunol. 2012 Mar 14;13:12. doi: 10.1186/1471-2172-13-12. BMC Immunol. 2012. PMID: 22413885 Free PMC article.
-
Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion.J Immunol. 2014 Aug 15;193(4):1525-30. doi: 10.4049/jimmunol.1400557. J Immunol. 2014. PMID: 25086175 Free PMC article. Review.
-
Regulation of NF-κB induction by TCR/CD28.Immunol Res. 2011 Aug;50(2-3):113-7. doi: 10.1007/s12026-011-8216-z. Immunol Res. 2011. PMID: 21717079 Free PMC article. Review.
Cited by
-
Phosphatidylserine-targeting antibodies augment the anti-tumorigenic activity of anti-PD-1 therapy by enhancing immune activation and downregulating pro-oncogenic factors induced by T-cell checkpoint inhibition in murine triple-negative breast cancers.Breast Cancer Res. 2016 May 11;18(1):50. doi: 10.1186/s13058-016-0708-2. Breast Cancer Res. 2016. PMID: 27169467 Free PMC article.
-
Not All Immune Checkpoints Are Created Equal.Front Immunol. 2018 Aug 31;9:1909. doi: 10.3389/fimmu.2018.01909. eCollection 2018. Front Immunol. 2018. PMID: 30233564 Free PMC article. Review.
-
A luminescence-based method to assess antigen presentation and antigen-specific T cell responses for in vitro screening of immunomodulatory checkpoints and therapeutics.Front Immunol. 2023 Jul 25;14:1233113. doi: 10.3389/fimmu.2023.1233113. eCollection 2023. Front Immunol. 2023. PMID: 37559730 Free PMC article.
-
B Cells versus T Cells in the Tumor Microenvironment of Malignant Lymphomas. Are the Lymphocytes Playing the Roles of Muhammad Ali versus George Foreman in Zaire 1974?J Clin Med. 2020 Oct 24;9(11):3412. doi: 10.3390/jcm9113412. J Clin Med. 2020. PMID: 33114418 Free PMC article. Review.
-
A Luminescence-Based Method for In Vitro Screening of Immunomodulatory Checkpoints and Therapeutics.Methods Mol Biol. 2025;2930:245-257. doi: 10.1007/978-1-0716-4558-1_17. Methods Mol Biol. 2025. PMID: 40402459
References
-
- Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(18):4917–24. 10.1158/1078-0432.CCR-12-1972 - DOI - PMC - PubMed
-
- Wherry EJ. T cell exhaustion. Nature immunology. 2011;12(6):492–9. . - PubMed
-
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science translational medicine. 2012;4(127):127ra37 10.1126/scitranslmed.3003689 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

