Comparative effectiveness of inhaled corticosteroids for paediatric asthma: protocol for a systematic review and Bayesian network meta-analysis
- PMID: 26493456
- PMCID: PMC4620167
- DOI: 10.1136/bmjopen-2015-008501
Comparative effectiveness of inhaled corticosteroids for paediatric asthma: protocol for a systematic review and Bayesian network meta-analysis
Abstract
Introduction: Use of inhaled corticosteroid (ICS) is the mainstream maintenance therapy for paediatric asthma. Several forms of ICS are available, but the relative effectiveness among ICS has not been well investigated in published, randomised, controlled trials. The paucity of direct comparisons between ICS may have resulted in insufficient estimation in former systematic reviews/meta-analyses. To supplement the information on the comparative effectiveness of ICS for paediatric asthma, we plan to conduct a network meta-analysis that will enable summary of direct and indirect evidence.
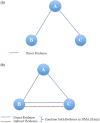
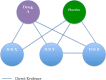
Methods and analysis: We will retrieve randomised, controlled trials that examined the effectiveness of ICS for paediatric asthma from the PubMed and Cochrane Central Register of Controlled Trials. After one author scans the title and abstract for eligible studies, two authors will independently review study data and assess the quality of the study. Studies of children (≤18 years old) with chronic asthma or recurrent wheezing episodes will be included if they used ICS for ≥4 weeks. We will define a priori core outcomes and supplemental outcomes of paediatric asthma, including exacerbation, healthcare use and pulmonary function. Studies reporting a minimum of one core outcome will be entered into the systematic review. After the systematic review is performed, extracted data of relevant studies will be synthesised in the Bayesian framework using a random-effects model.
Ethics and dissemination: The results will be disseminated through peer-reviewed publications and conference presentations.
Protocol registration number: UMIN (000016724) and PROSPERO (CRD42015025889).
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
Similar articles
-
Daily inhaled corticosteroids or montelukast for preschoolers with asthma or recurrent wheezing: A systematic review.Pediatr Pulmonol. 2018 Dec;53(12):1670-1677. doi: 10.1002/ppul.24176. Epub 2018 Nov 5. Pediatr Pulmonol. 2018. PMID: 30394700
-
Predictors of treatment REsponse to inhaled corticosteroids (ICS) in Chronic Obstructive pulmonary disease: randomised controlled trials individual participant Data re-Evaluation-protocol of the ICS-RECODE individual participant data meta-analysis.BMJ Open. 2025 Mar 5;15(3):e095541. doi: 10.1136/bmjopen-2024-095541. BMJ Open. 2025. PMID: 40044194 Free PMC article.
-
Comparison of leukotriene receptor antagonists in addition to inhaled corticosteroid and inhaled corticosteroid alone in the treatment of adolescents and adults with bronchial asthma: a meta-analysis.Asian Pac J Allergy Immunol. 2012 Jun;30(2):130-8. Asian Pac J Allergy Immunol. 2012. PMID: 22830292
-
Inhaled corticosteroids for the prevention of asthma exacerbations.Ann Allergy Asthma Immunol. 2021 Nov;127(5):524-529. doi: 10.1016/j.anai.2021.08.014. Epub 2021 Aug 13. Ann Allergy Asthma Immunol. 2021. PMID: 34400314 Free PMC article. Review.
-
The effectiveness of inhaled corticosteroids in the emergency department treatment of acute asthma: a meta-analysis.Ann Emerg Med. 2002 Aug;40(2):145-54. doi: 10.1067/mem.2002.124753. Ann Emerg Med. 2002. PMID: 12140492
Cited by
-
Respiratory pathogens and clinical outcomes in children with an asthma exacerbation: A systematic review.J Assoc Med Microbiol Infect Dis Can. 2019 Oct 11;4(3):145-168. doi: 10.3138/jammi.2019-0004. eCollection 2019 Oct. J Assoc Med Microbiol Infect Dis Can. 2019. PMID: 36340656 Free PMC article. Review.
-
Differences in the effectiveness of single, dual, and triple inhaled corticosteroid therapy for reducing future risk of severe asthma exacerbation: A systematic review and network meta-analysis.Heliyon. 2024 May 16;10(12):e31186. doi: 10.1016/j.heliyon.2024.e31186. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39022061 Free PMC article.
References
-
- Guilbert T, Moss HM, Lemanske RF Jr. Approach to infants and children with asthma. In; Adkinson NF Jr, Bochner BS, Busse WW et al.. eds Middleton's allergy: principles and practice. Philadelphia; Saunders, 2009:1319–44.
-
- Centers for Disease Control and Prevention. CDC vital signs: Asthma in the US 2011. http://www.cdc.gov/vitalsigns/pdf/2011–05-vitalsigns.pdf (accessed 16 Mar 2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
