Efficacy of Anodal Transcranial Direct Current Stimulation is Related to Sensitivity to Transcranial Magnetic Stimulation
- PMID: 26493498
- PMCID: PMC4724228
- DOI: 10.1016/j.brs.2015.08.014
Efficacy of Anodal Transcranial Direct Current Stimulation is Related to Sensitivity to Transcranial Magnetic Stimulation
Abstract
Background: Transcranial direct current stimulation (tDCS) has become an important non-invasive brain stimulation tool for basic human brain physiology and cognitive neuroscience, with potential applications in cognitive and motor rehabilitation. To date, tDCS studies have employed a fixed stimulation level, without considering the impact of individual anatomy and physiology on the efficacy of the stimulation. This approach contrasts with the standard procedure for transcranial magnetic stimulation (TMS) where stimulation levels are usually tailored on an individual basis.
Objective/hypothesis: The present study tests whether the efficacy of tDCS-induced changes in corticospinal excitability varies as a function of individual differences in sensitivity to TMS.
Methods: We performed an archival review to examine the relationship between the TMS intensity required to induce 1 mV motor-evoked potentials (MEPs) and the efficacy of (fixed-intensity) tDCS over the primary motor cortex (M1). For the latter, we examined tDCS-induced changes in corticospinal excitability, operationalized by comparing MEPs before and after anodal or cathodal tDCS. For comparison, we performed a similar analysis on data sets in which MEPs had been obtained before and after paired associative stimulation (PAS), a non-invasive brain stimulation technique in which the stimulation intensity is adjusted on an individual basis.
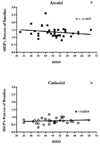
Results: MEPs were enhanced following anodal tDCS. This effect was larger in participants more sensitive to TMS as compared to those less sensitive to TMS, with sensitivity defined as the TMS intensity required to produce MEPs amplitudes of the size of 1 mV. While MEPs were attenuated following cathodal tDCS, the magnitude of this attenuation was not related to TMS sensitivity nor was there a relationship between TMS sensitivity and responsiveness to PAS.
Conclusion: Accounting for variation in individual sensitivity to non-invasive brain stimulation may enhance the utility of tDCS as a tool for understanding brain-behavior interactions and as a method for clinical interventions.
Keywords: Intensity; TMS; Variability; tDCS.
Published by Elsevier Inc.
Figures




Similar articles
-
Variability in response to transcranial direct current stimulation of the motor cortex.Brain Stimul. 2014 May-Jun;7(3):468-75. doi: 10.1016/j.brs.2014.02.003. Epub 2014 Feb 15. Brain Stimul. 2014. PMID: 24630848 Clinical Trial.
-
Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation.J Physiol. 2017 Feb 15;595(4):1273-1288. doi: 10.1113/JP272738. Epub 2016 Nov 8. J Physiol. 2017. PMID: 27723104 Free PMC article.
-
Excitability modulation of the motor system induced by transcranial direct current stimulation: a multimodal approach.Neuroimage. 2013 Dec;83:569-80. doi: 10.1016/j.neuroimage.2013.06.076. Epub 2013 Jul 9. Neuroimage. 2013. PMID: 23845429
-
Transcranial Magnetic and Direct Current Stimulation (TMS/tDCS) for the Treatment of Headache: A Systematic Review.Headache. 2019 Mar;59(3):339-357. doi: 10.1111/head.13479. Epub 2019 Jan 23. Headache. 2019. PMID: 30671941
-
A Review of Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation Combined with Medication and Psychotherapy for Depression.Harv Rev Psychiatry. 2024 May-Jun 01;32(3):77-95. doi: 10.1097/HRP.0000000000000396. Harv Rev Psychiatry. 2024. PMID: 38728568 Review.
Cited by
-
Transcranial Direct Current Stimulation of Primary Motor Cortex over Multiple Days Improves Motor Learning of a Complex Overhand Throwing Task.Brain Sci. 2023 Oct 10;13(10):1441. doi: 10.3390/brainsci13101441. Brain Sci. 2023. PMID: 37891809 Free PMC article.
-
Probing Our Built-in Calculator: A Systematic Narrative Review of Noninvasive Brain Stimulation Studies on Arithmetic Operation-Related Brain Areas.eNeuro. 2024 Apr 5;11(4):ENEURO.0318-23.2024. doi: 10.1523/ENEURO.0318-23.2024. Print 2024 Apr. eNeuro. 2024. PMID: 38580452 Free PMC article.
-
Current intensity- and polarity-specific online and aftereffects of transcranial direct current stimulation: An fMRI study.Hum Brain Mapp. 2020 Apr 15;41(6):1644-1666. doi: 10.1002/hbm.24901. Epub 2019 Dec 20. Hum Brain Mapp. 2020. PMID: 31860160 Free PMC article.
-
The Influence of Transcranial Direct Current Stimulation on Shooting Performance in Elite Deaflympic Athletes: A Case Series.J Funct Morphol Kinesiol. 2022 May 25;7(2):42. doi: 10.3390/jfmk7020042. J Funct Morphol Kinesiol. 2022. PMID: 35736013 Free PMC article.
-
Dose-Response Transcranial Electrical Stimulation Study Design: A Well-Controlled Adaptive Seamless Bayesian Method to Illuminate Negative Valence Role in Tinnitus Perception.Front Hum Neurosci. 2022 May 12;16:811550. doi: 10.3389/fnhum.2022.811550. eCollection 2022. Front Hum Neurosci. 2022. PMID: 35677206 Free PMC article.
References
-
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain J Neurol. 2000;123(Pt 3):572–584. - PubMed
-
- Ferrucci R, Priori A. Transcranial cerebellar direct current stimulation (tcDCS): motor control, cognition, learning and emotions. NeuroImage. 2014;85(Pt 3):918–923. - PubMed
-
- Kuo M-F, Paulus W, Nitsche MA. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage. 2014;85(Pt 3):948–960. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

