Caffeine stimulates locomotor activity in the mammalian spinal cord via adenosine A1 receptor-dopamine D1 receptor interaction and PKA-dependent mechanisms
- PMID: 26493631
- PMCID: PMC4855515
- DOI: 10.1016/j.neuropharm.2015.10.020
Caffeine stimulates locomotor activity in the mammalian spinal cord via adenosine A1 receptor-dopamine D1 receptor interaction and PKA-dependent mechanisms
Abstract
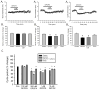
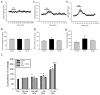
Caffeine is a potent psychostimulant that can have significant and widely variable effects on the activity of multiple neuronal pathways. The most pronounced caffeine-induced behavioral effect seen in rodents is to increase locomotor activity which has been linked to a dose-dependent inhibition of A1 and A(2A) receptors. The effects of caffeine at the level of the lumbar spinal central pattern generator (CPG) network for hindlimb locomotion are lacking. We assessed the effects of caffeine to the locomotor function of the spinal CPG network via extracellular ventral root recordings using the isolated neonatal mouse spinal cord preparation. Addition of caffeine and of an A1 receptor antagonist significantly decreased the cycle period accelerating the ongoing locomotor rhythm, while decreasing burst duration reversibly in most preparations suggesting the role of A1 receptors as the primary target of caffeine. Caffeine and an A1 receptor antagonist failed to stimulate ongoing locomotor activity in the absence of dopamine or in the presence of a D1 receptor antagonist supporting A1/D1 receptor-dependent mechanism of action. The use of caffeine or an A1 receptor blocker failed to stimulate an ongoing locomotor rhythm in the presence of a blocker of the cAMP-dependent protein kinase (PKA) supporting the need of this intracellular pathway for the modulatory effects of caffeine to occur. These results support a stimulant effect of caffeine on the lumbar spinal network controlling hindlimb locomotion through the inhibition of A1 receptors and subsequent activation of D1 receptors via a PKA-dependent intracellular mechanism.
Keywords: Adenosine; Caffeine; Dopamine; Locomotion; Mouse; Spinal cord.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

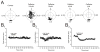



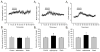



References
-
- Agnati LF, Ferré S, Lluis C, Franco R, Fuxe K. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev. 2003;55:509–550. - PubMed
-
- Antoniou K, Papadopoulou-Daifoti Z, Hyphantis T, Papathanasiou G, Bekris E, Marselos M, Panlilio L, Müller CE, Goldberg SR, Ferré S. A detailed behavioral analysis of the acute motor effects of caffeine in the rat: involvement of adenosine A1 and A2A receptors. Psychopharmacology (Berl) 2005;183(2):154–62. - PubMed
-
- Barraco RA, Martens KA, Parizon M, Normile HJ. Role of adenosine A2A receptors in the nucleus accumbens. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18(3):545–53. - PubMed
-
- Bedingfield JB, King DA, Holloway FA. Cocaine and caffeine: conditioned place preference, locomotor activity, and additivity. Pharmacol Biochem Behav. 1998;61:291–296. - PubMed
-
- Beninger RJ, Miller R. Dopamine D1-like receptors and reward-related incentive learning. Neurosci Biobehav Rev. 1998;22(2):335–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

