Complement, a target for therapy in inflammatory and degenerative diseases
- PMID: 26493766
- PMCID: PMC7098197
- DOI: 10.1038/nrd4657
Complement, a target for therapy in inflammatory and degenerative diseases
Abstract
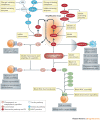
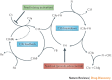
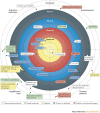
The complement system is a key innate immune defence against infection and an important driver of inflammation; however, these very properties can also cause harm. Inappropriate or uncontrolled activation of complement can cause local and/or systemic inflammation, tissue damage and disease. Complement provides numerous options for drug development as it is a proteolytic cascade that involves nine specific proteases, unique multimolecular activation and lytic complexes, an arsenal of natural inhibitors, and numerous receptors that bind to activation fragments. Drug design is facilitated by the increasingly detailed structural understanding of the molecules involved in the complement system. Only two anti-complement drugs are currently on the market, but many more are being developed for diseases that include infectious, inflammatory, degenerative, traumatic and neoplastic disorders. In this Review, we describe the history, current landscape and future directions for anti-complement therapies.
Conflict of interest statement
B.P.M. has undertaken paid consultancy work and sponsored research for GlaxoSmithKline, Swedish Orphan Biovitrum and Achillion Pharma. C.L.H. is employed by GlaxoSmithKline.
Figures
Similar articles
-
Complement in therapy and disease: Regulating the complement system with antibody-based therapeutics.Mol Immunol. 2015 Oct;67(2 Pt A):117-30. doi: 10.1016/j.molimm.2015.01.028. Epub 2015 Feb 17. Mol Immunol. 2015. PMID: 25697848 Review.
-
Complement: A primer for the coming therapeutic revolution.Pharmacol Ther. 2017 Apr;172:63-72. doi: 10.1016/j.pharmthera.2016.11.014. Epub 2016 Dec 1. Pharmacol Ther. 2017. PMID: 27914981 Review.
-
Tissue-targeted complement therapeutics.Mol Immunol. 2018 Oct;102:120-128. doi: 10.1016/j.molimm.2018.06.005. Epub 2018 Jul 7. Mol Immunol. 2018. PMID: 30220307 Free PMC article. Review.
-
Clinical promise of next-generation complement therapeutics.Nat Rev Drug Discov. 2019 Sep;18(9):707-729. doi: 10.1038/s41573-019-0031-6. Epub 2019 Jul 19. Nat Rev Drug Discov. 2019. PMID: 31324874 Free PMC article. Review.
-
Therapeutic potential of complement modulation.Nat Rev Drug Discov. 2010 Jan;9(1):43-56. doi: 10.1038/nrd3011. Epub 2009 Dec 4. Nat Rev Drug Discov. 2010. PMID: 19960015 Review.
Cited by
-
Discovery of functionally distinct anti-C7 monoclonal antibodies and stratification of anti-nicotinic AChR positive Myasthenia Gravis patients.Front Immunol. 2022 Sep 5;13:968206. doi: 10.3389/fimmu.2022.968206. eCollection 2022. Front Immunol. 2022. PMID: 36148231 Free PMC article.
-
Structural Basis for Simvastatin Competitive Antagonism of Complement Receptor 3.J Biol Chem. 2016 Aug 12;291(33):16963-76. doi: 10.1074/jbc.M116.732222. Epub 2016 Jun 23. J Biol Chem. 2016. PMID: 27339893 Free PMC article.
-
Impact of Preanalytical Procedures on Complement Biomarkers in Cerebrospinal Fluid and Plasma from Controls and Alzheimer's Disease Patients.J Alzheimers Dis. 2024;101(2):563-576. doi: 10.3233/JAD-240287. J Alzheimers Dis. 2024. PMID: 39213066 Free PMC article.
-
Heart disease in a mutant mouse model of spontaneous eosinophilic myocarditis maps to three loci.BMC Genomics. 2019 Oct 11;20(1):727. doi: 10.1186/s12864-019-6108-0. BMC Genomics. 2019. PMID: 31601172 Free PMC article.
-
Spinning convincing stories for both true and false association signals.Genet Epidemiol. 2019 Jun;43(4):356-364. doi: 10.1002/gepi.22189. Epub 2019 Jan 18. Genet Epidemiol. 2019. PMID: 30657194 Free PMC article. Review.
References
-
- Morgan BP. Fundamental Immunology. 2012. pp. 850–862.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

