Dopamine Prediction Errors in Reward Learning and Addiction: From Theory to Neural Circuitry
- PMID: 26494275
- PMCID: PMC4760620
- DOI: 10.1016/j.neuron.2015.08.037
Dopamine Prediction Errors in Reward Learning and Addiction: From Theory to Neural Circuitry
Abstract
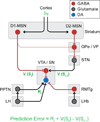
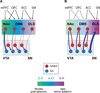
Midbrain dopamine (DA) neurons are proposed to signal reward prediction error (RPE), a fundamental parameter in associative learning models. This RPE hypothesis provides a compelling theoretical framework for understanding DA function in reward learning and addiction. New studies support a causal role for DA-mediated RPE activity in promoting learning about natural reward; however, this question has not been explicitly tested in the context of drug addiction. In this review, we integrate theoretical models with experimental findings on the activity of DA systems, and on the causal role of specific neuronal projections and cell types, to provide a circuit-based framework for probing DA-RPE function in addiction. By examining error-encoding DA neurons in the neural network in which they are embedded, hypotheses regarding circuit-level adaptations that possibly contribute to pathological error signaling and addiction can be formulated and tested.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- Aggarwal M, Hyland BI, Wickens JR. Neural control of dopamine neurotransmission: Implications for reinforcement learning. Eur. J. Neurosci. 2012;35:1115–1123. - PubMed
-
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. - PubMed
-
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

