Fetal endocannabinoids orchestrate the organization of pancreatic islet microarchitecture
- PMID: 26494286
- PMCID: PMC4653226
- DOI: 10.1073/pnas.1519040112
Fetal endocannabinoids orchestrate the organization of pancreatic islet microarchitecture
Abstract
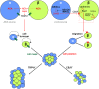
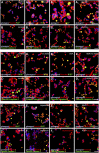
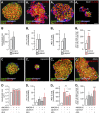
Endocannabinoids are implicated in the control of glucose utilization and energy homeostasis by orchestrating pancreatic hormone release. Moreover, in some cell niches, endocannabinoids regulate cell proliferation, fate determination, and migration. Nevertheless, endocannabinoid contributions to the development of the endocrine pancreas remain unknown. Here, we show that α cells produce the endocannabinoid 2-arachidonoylglycerol (2-AG) in mouse fetuses and human pancreatic islets, which primes the recruitment of β cells by CB1 cannabinoid receptor (CB1R) engagement. Using subtractive pharmacology, we extend these findings to anandamide, a promiscuous endocannabinoid/endovanilloid ligand, which impacts both the determination of islet size by cell proliferation and α/β cell sorting by differential activation of transient receptor potential cation channel subfamily V member 1 (TRPV1) and CB1Rs. Accordingly, genetic disruption of TRPV1 channels increases islet size whereas CB1R knockout augments cellular heterogeneity and favors insulin over glucagon release. Dietary enrichment in ω-3 fatty acids during pregnancy and lactation in mice, which permanently reduces endocannabinoid levels in the offspring, phenocopies CB1R(-/-) islet microstructure and improves coordinated hormone secretion. Overall, our data mechanistically link endocannabinoids to cell proliferation and sorting during pancreatic islet formation, as well as to life-long programming of hormonal determinants of glucose homeostasis.
Keywords: G protein-coupled receptor; cell adhesion; diabetes; migration; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Effects of endocannabinoid and endovanilloid systems on aversive memory extinction.Behav Brain Res. 2013 Nov 1;256:101-7. doi: 10.1016/j.bbr.2013.08.010. Epub 2013 Aug 12. Behav Brain Res. 2013. PMID: 23948212
-
Altered responses of dopamine D3 receptor null mice to excitotoxic or anxiogenic stimuli: Possible involvement of the endocannabinoid and endovanilloid systems.Neurobiol Dis. 2009 Oct;36(1):70-80. doi: 10.1016/j.nbd.2009.06.015. Epub 2009 Jul 8. Neurobiol Dis. 2009. PMID: 19591935
-
Endocannabinoids in the Islets of Langerhans: the ugly, the bad, and the good facts.Am J Physiol Endocrinol Metab. 2018 Aug 1;315(2):E174-E179. doi: 10.1152/ajpendo.00338.2017. Epub 2018 Apr 6. Am J Physiol Endocrinol Metab. 2018. PMID: 29631361 Free PMC article. Review.
-
Endocannabinoids and endocannabinoid-like compounds modulate hypoxia-induced permeability in CaCo-2 cells via CB1, TRPV1, and PPARα.Biochem Pharmacol. 2019 Oct;168:465-472. doi: 10.1016/j.bcp.2019.07.017. Epub 2019 Jul 17. Biochem Pharmacol. 2019. PMID: 31325449
-
The role of the endocannabinoid system in islet biology.Curr Opin Endocrinol Diabetes Obes. 2011 Apr;18(2):153-8. doi: 10.1097/MED.0b013e32834455a8. Curr Opin Endocrinol Diabetes Obes. 2011. PMID: 21311323 Review.
Cited by
-
RISING STARS: Mechanistic insights into maternal-fetal cross talk and islet beta-cell development.J Endocrinol. 2023 Nov 8;259(3):e230069. doi: 10.1530/JOE-23-0069. Print 2023 Dec 1. J Endocrinol. 2023. PMID: 37855321 Free PMC article. Review.
-
Anti-Cancer and Anti-Proliferative Potential of Cannabidiol: A Cellular and Molecular Perspective.Int J Mol Sci. 2024 May 23;25(11):5659. doi: 10.3390/ijms25115659. Int J Mol Sci. 2024. PMID: 38891847 Free PMC article. Review.
-
Functional Fine-Tuning of Metabolic Pathways by the Endocannabinoid System-Implications for Health and Disease.Int J Mol Sci. 2021 Apr 1;22(7):3661. doi: 10.3390/ijms22073661. Int J Mol Sci. 2021. PMID: 33915889 Free PMC article. Review.
-
Countering the Modern Metabolic Disease Rampage With Ancestral Endocannabinoid System Alignment.Front Endocrinol (Lausanne). 2019 May 17;10:311. doi: 10.3389/fendo.2019.00311. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31156558 Free PMC article.
-
Exposure to Δ9-tetrahydrocannabinol during rat pregnancy leads to impaired cardiac dysfunction in postnatal life.Pediatr Res. 2021 Sep;90(3):532-539. doi: 10.1038/s41390-021-01511-9. Epub 2021 Apr 20. Pediatr Res. 2021. PMID: 33879850 Free PMC article.
References
-
- Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab. 2007;18(1):27–37. - PubMed
-
- Li C, et al. Cannabinoid receptor agonists and antagonists stimulate insulin secretion from isolated human islets of Langerhans. Diabetes Obes Metab. 2011;13(10):903–910. - PubMed
-
- Vilches-Flores A, Hauge-Evans AC, Jones PM, Persaud SJ. Chronic activation of cannabinoid receptors in vitro does not compromise mouse islet function. Clin Sci (Lond) 2013;124(7):467–478. - PubMed
-
- Li C, et al. Expression and function of monoacylglycerol lipase in mouse β-cells and human islets of Langerhans. Cell Physiol Biochem. 2012;30(2):347–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

