Targeting truncated RXRα for cancer therapy
- PMID: 26494413
- PMCID: PMC4689158
- DOI: 10.1093/abbs/gmv104
Targeting truncated RXRα for cancer therapy
Abstract
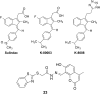
Retinoid X receptor-alpha (RXRα), a unique member of the nuclear receptor superfamily, is a well-established drug target, representing one of the most important targets for pharmacologic interventions and therapeutic applications for cancer. However, how RXRα regulates cancer cell growth and how RXRα modulators suppress tumorigenesis are poorly understood. Altered expression and aberrant function of RXRα are implicated in the development of cancer. Previously, several studies had demonstrated the presence of N-terminally truncated RXRα (tRXRα) proteins resulted from limited proteolysis of RXRα in tumor cells. Recently, we discovered that overexpression of tRXRα can promote tumor growth by interacting with tumor necrosis factor-alpha-induced phosphoinositide 3-kinase and NF-κB signal transduction pathways. We also identified nonsteroidal anti-inflammatory drug Sulindac and analogs as effective inhibitors of tRXRα activities via a unique binding mechanism. This review discusses the emerging roles of tRXRα and modulators in the regulation of cancer cell survival and death as well as inflammation and our recent understanding of tRXRα regulation by targeting the alternate binding sites on its surface.
Keywords: PI3K; RXRα modulators; inflammation; nongenomic action; tRXRα.
© The Author 2015. Published by ABBS Editorial Office in association with Oxford University Press on behalf of the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
Figures



Similar articles
-
Overview of the structure-based non-genomic effects of the nuclear receptor RXRα.Cell Mol Biol Lett. 2018 Aug 7;23:36. doi: 10.1186/s11658-018-0103-3. eCollection 2018. Cell Mol Biol Lett. 2018. PMID: 30093910 Free PMC article. Review.
-
NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling.Cancer Cell. 2010 Jun 15;17(6):560-73. doi: 10.1016/j.ccr.2010.04.023. Cancer Cell. 2010. PMID: 20541701 Free PMC article.
-
Antagonist effect of triptolide on AKT activation by truncated retinoid X receptor-alpha.PLoS One. 2012;7(4):e35722. doi: 10.1371/journal.pone.0035722. Epub 2012 Apr 24. PLoS One. 2012. PMID: 22545132 Free PMC article.
-
Modulation of nongenomic activation of PI3K signalling by tetramerization of N-terminally-cleaved RXRα.Nat Commun. 2017 Jul 17;8:16066. doi: 10.1038/ncomms16066. Nat Commun. 2017. PMID: 28714476 Free PMC article.
-
Regulation of the nongenomic actions of retinoid X receptor-α by targeting the coregulator-binding sites.Acta Pharmacol Sin. 2015 Jan;36(1):102-12. doi: 10.1038/aps.2014.109. Epub 2014 Dec 1. Acta Pharmacol Sin. 2015. PMID: 25434990 Free PMC article. Review.
Cited by
-
Structural overview and perspectives of the nuclear receptors, a major family as the direct targets for small-molecule drugs.Acta Biochim Biophys Sin (Shanghai). 2022 Jan 25;54(1):12-24. doi: 10.3724/abbs.2021001. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 35130630 Free PMC article.
-
The role of resveratrol, Sirtuin1 and RXRα as prognostic markers in ovarian cancer.Arch Gynecol Obstet. 2022 Jun;305(6):1559-1572. doi: 10.1007/s00404-021-06262-w. Epub 2021 Dec 6. Arch Gynecol Obstet. 2022. PMID: 34870752 Free PMC article.
-
Celastrol-regulated gut microbiota and bile acid metabolism alleviate hepatocellular carcinoma proliferation by regulating the interaction between FXR and RXRα in vivo and in vitro.Front Pharmacol. 2023 Feb 15;14:1124240. doi: 10.3389/fphar.2023.1124240. eCollection 2023. Front Pharmacol. 2023. PMID: 36874033 Free PMC article.
-
Overview of the structure-based non-genomic effects of the nuclear receptor RXRα.Cell Mol Biol Lett. 2018 Aug 7;23:36. doi: 10.1186/s11658-018-0103-3. eCollection 2018. Cell Mol Biol Lett. 2018. PMID: 30093910 Free PMC article. Review.
-
Oncogenic potential of truncated RXRα during colitis-associated colorectal tumorigenesis by promoting IL-6-STAT3 signaling.Nat Commun. 2019 Apr 1;10(1):1463. doi: 10.1038/s41467-019-09375-8. Nat Commun. 2019. PMID: 30931933 Free PMC article.
References
-
- Szanto A, Narkar V, Shen Q, Uray IP, Davies PJ, Nagy L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ 2004, 11(Suppl 2): S126–S143. - PubMed
-
- Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov 2007, 6: 793–810. - PubMed
-
- Dawson MI, Zhang XK. Discovery and design of retinoic acid receptor and retinoid X receptor class- and subtype-selective synthetic analogs of all-trans-retinoic acid and 9-cis-retinoic acid. Curr Med Chem 2002, 9: 623–637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

