Lactobacillus plantarum displaying CCL3 chemokine in fusion with HIV-1 Gag derived antigen causes increased recruitment of T cells
- PMID: 26494531
- PMCID: PMC4618854
- DOI: 10.1186/s12934-015-0360-z
Lactobacillus plantarum displaying CCL3 chemokine in fusion with HIV-1 Gag derived antigen causes increased recruitment of T cells
Abstract
Background: Chemokines are attractive candidates for vaccine adjuvants due to their ability to recruit the immune cells. Lactic acid bacteria (LAB)-based delivery vehicles have potential to be used as a cheap and safe option for vaccination. Chemokine produced on the surface of LAB may potentially enhance the immune response to an antigen and this approach can be considered in development of future mucosal vaccines.
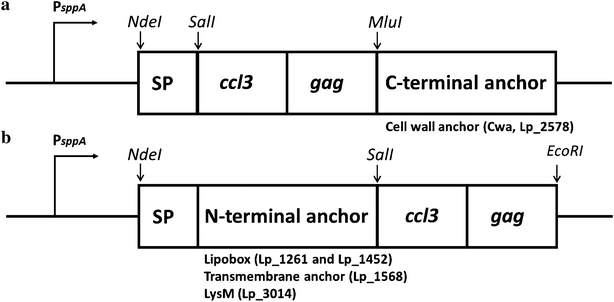
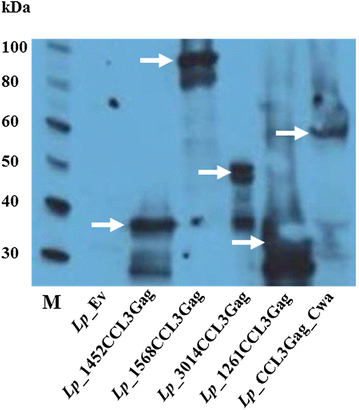
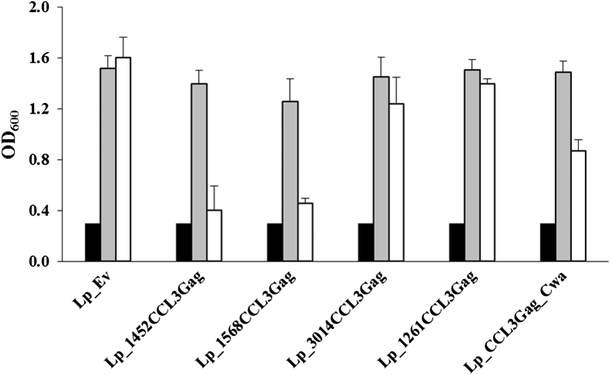
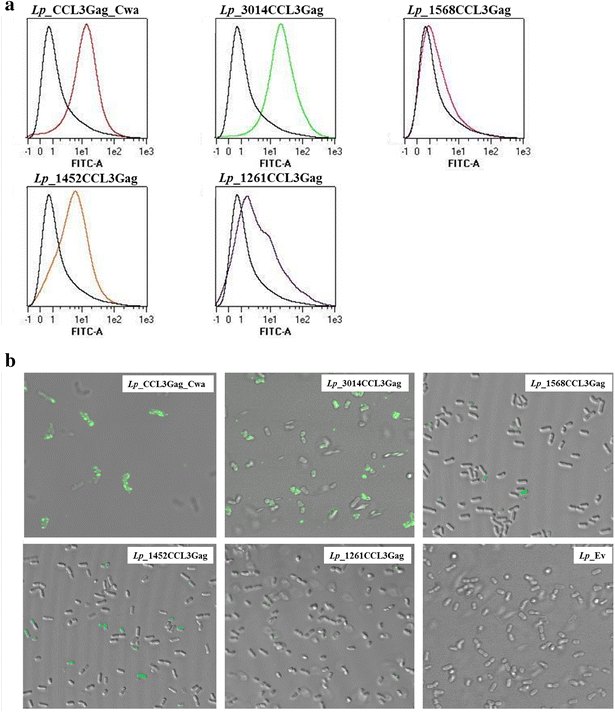
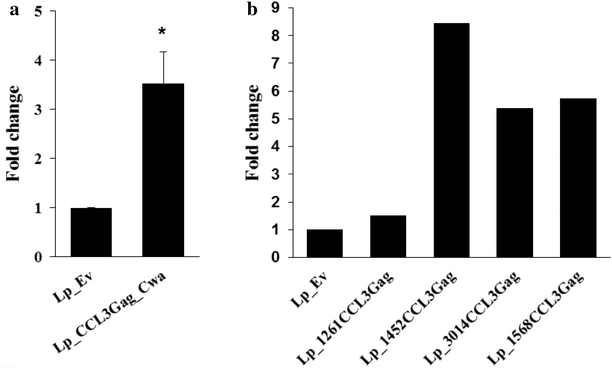
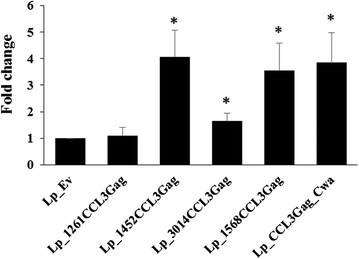
Results: We have constructed strains of Lactobacillus plantarum displaying a chemokine on their surface. L. plantarum was genetically engineered to express and anchor to the surface a protein called CCL3Gag. CCL3Gag is a fusion protein comprising of truncated HIV-1 Gag antigen and the murine chemokine CCL3, also known as MIP-1α. Various surface anchoring strategies were explored: (1) a lipobox-based covalent membrane anchor, (2) sortase-mediated covalent cell wall anchoring, (3) LysM-based non-covalent cell wall anchoring, and (4) an N-terminal signal peptide-based transmembrane anchor. Protein production and correct localization were confirmed using Western blotting, flow cytometry and immunofluorescence microscopy. Using a chemotaxis assay, we demonstrated that CCL3Gag-producing L. plantarum strains are able to recruit immune cells in vitro.
Conclusions: The results show the ability of engineered L. plantarum to produce a functional chemotactic protein immobilized on the bacterial surface. We observed that the activity of surface-displayed CCL3Gag differed depending on the type of anchor used. The chemokine which is a part of the bacteria-based vaccine may increase the recruitment of immune cells and, thereby, enhance the reaction of the immune system to the vaccine.
Figures
Similar articles
-
Immunogenic Properties of Lactobacillus plantarum Producing Surface-Displayed Mycobacterium tuberculosis Antigens.Appl Environ Microbiol. 2016 Dec 30;83(2):e02782-16. doi: 10.1128/AEM.02782-16. Print 2017 Jan 15. Appl Environ Microbiol. 2016. PMID: 27815271 Free PMC article.
-
Surface display of an anti-DEC-205 single chain Fv fragment in Lactobacillus plantarum increases internalization and plasmid transfer to dendritic cells in vitro and in vivo.Microb Cell Fact. 2015 Jul 4;14:95. doi: 10.1186/s12934-015-0290-9. Microb Cell Fact. 2015. PMID: 26141059 Free PMC article.
-
Anchoring of heterologous proteins in multiple Lactobacillus species using anchors derived from Lactobacillus plantarum.Sci Rep. 2020 Jun 15;10(1):9640. doi: 10.1038/s41598-020-66531-7. Sci Rep. 2020. PMID: 32541679 Free PMC article.
-
Display of a β-mannanase and a chitosanase on the cell surface of Lactobacillus plantarum towards the development of whole-cell biocatalysts.Microb Cell Fact. 2016 Oct 4;15(1):169. doi: 10.1186/s12934-016-0570-z. Microb Cell Fact. 2016. PMID: 27716231 Free PMC article.
-
Display of recombinant proteins at the surface of lactic acid bacteria: strategies and applications.Microb Cell Fact. 2016 May 3;15:70. doi: 10.1186/s12934-016-0468-9. Microb Cell Fact. 2016. PMID: 27142045 Free PMC article. Review.
Cited by
-
Protective effects of a food-grade recombinant Lactobacillus plantarum with surface displayed AMA1 and EtMIC2 proteins of Eimeria tenella in broiler chickens.Microb Cell Fact. 2020 Feb 11;19(1):28. doi: 10.1186/s12934-020-1297-4. Microb Cell Fact. 2020. PMID: 32046719 Free PMC article.
-
Lactiplantibacillus plantarum: a new example of inclusion body producing bacteria.Microb Cell Fact. 2023 Jun 9;22(1):111. doi: 10.1186/s12934-023-02120-3. Microb Cell Fact. 2023. PMID: 37296442 Free PMC article.
-
Applications of chemokines as adjuvants for vaccine immunotherapy.Immunobiology. 2018 Jun-Jul;223(6-7):477-485. doi: 10.1016/j.imbio.2017.12.001. Epub 2017 Dec 8. Immunobiology. 2018. PMID: 29246401 Free PMC article. Review.
-
Immobilization of β-Galactosidases on the Lactobacillus Cell Surface Using the Peptidoglycan-Binding Motif LysM.Catalysts. 2019 May;9(5):443. doi: 10.3390/catal9050443. Catalysts. 2019. PMID: 31595189 Free PMC article.
-
The Role of Mucosal Immunity and Recombinant Probiotics in SARS-CoV2 Vaccine Development.Probiotics Antimicrob Proteins. 2021 Oct;13(5):1239-1253. doi: 10.1007/s12602-021-09773-9. Epub 2021 Mar 26. Probiotics Antimicrob Proteins. 2021. PMID: 33770348 Free PMC article. Review.
References
-
- Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

