Enhancing Autophagy with Drugs or Lung-directed Gene Therapy Reverses the Pathological Effects of Respiratory Epithelial Cell Proteinopathy
- PMID: 26494620
- PMCID: PMC4705969
- DOI: 10.1074/jbc.M115.691253
Enhancing Autophagy with Drugs or Lung-directed Gene Therapy Reverses the Pathological Effects of Respiratory Epithelial Cell Proteinopathy
Abstract
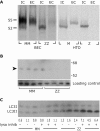
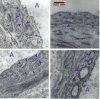
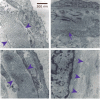
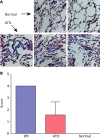
Recent studies have shown that autophagy mitigates the pathological effects of proteinopathies in the liver, heart, and skeletal muscle but this has not been investigated for proteinopathies that affect the lung. This may be due at least in part to the lack of an animal model robust enough for spontaneous pathological effects from proteinopathies even though several rare proteinopathies, surfactant protein A and C deficiencies, cause severe pulmonary fibrosis. In this report we show that the PiZ mouse, transgenic for the common misfolded variant α1-antitrypsin Z, is a model of respiratory epithelial cell proteinopathy with spontaneous pulmonary fibrosis. Intracellular accumulation of misfolded α1-antitrypsin Z in respiratory epithelial cells of the PiZ model resulted in activation of autophagy, leukocyte infiltration, and spontaneous pulmonary fibrosis severe enough to elicit functional restrictive deficits. Treatment with autophagy enhancer drugs or lung-directed gene transfer of TFEB, a master transcriptional activator of the autophagolysosomal system, reversed these proteotoxic consequences. We conclude that this mouse is an excellent model of respiratory epithelial proteinopathy with spontaneous pulmonary fibrosis and that autophagy is an important endogenous proteostasis mechanism and an attractive target for therapy.
Keywords: autophagy; chronic obstructive pulmonary disease (COPD); misfolded α1antitrypsin; protein misfolding; proteostasis; pulmonary fibrosis; α1antitrypsin deficiency.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
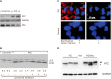

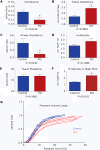
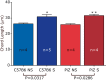
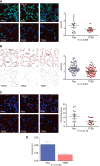


References
-
- Perlmutter D. H. (2011) α-1-Antitrypsin deficiency: importance of proteasomal and autophagic degradative pathways in disposal of liver disease-associated protein aggregates. Annu. Rev. Med. 62, 333–345 - PubMed
-
- Doppler K., Mittelbronn M., Lindner A., and Bornemann A. (2009) Basement membrane remodeling and segmental fibrosis in sporadic inclusion body myositis. Neuromuscul. Disord. 19, 406–411 - PubMed
-
- Zhong Q., Zhou B., Ann D. K., Minoo P., Liu Y., Banfalvi A., Krishnaveni M. S., Dubourd M., Demaio L., Willis B. C., Kim K. J., duBois R. M., Crandall E. D., Beers M. F., and Borok Z. (2011) Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am. J. Respir. Cell Mol. Biol. 45, 498–509 - PMC - PubMed
-
- Lawson W. E., Cheng D. S., Degryse A. L., Tanjore H., Polosukhin V. V., Xu X. C., Newcomb D. C., Jones B. R., Roldan J., Lane K. B., Morrisey E. E., Beers M. F., Yull F. E., and Blackwell T. S. (2011) Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc. Natl. Acad. Sci. U.S.A. 108, 10562–10567 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- P30DK072506/DK/NIDDK NIH HHS/United States
- HL113655/HL/NHLBI NIH HHS/United States
- P30 DK072506/DK/NIDDK NIH HHS/United States
- R01 DK084512/DK/NIDDK NIH HHS/United States
- HL037884/HL/NHLBI NIH HHS/United States
- DK096990/DK/NIDDK NIH HHS/United States
- R01 DK079806/DK/NIDDK NIH HHS/United States
- DK079806/DK/NIDDK NIH HHS/United States
- P01 DK096990/DK/NIDDK NIH HHS/United States
- TGM11MT3/TI_/Telethon/Italy
- DK084512/DK/NIDDK NIH HHS/United States
- AI01953/AI/NIAID NIH HHS/United States
- R01 HL113655/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

