Stability of small ubiquitin-like modifier (SUMO) proteases OVERLY TOLERANT TO SALT1 and -2 modulates salicylic acid signalling and SUMO1/2 conjugation in Arabidopsis thaliana
- PMID: 26494731
- PMCID: PMC4682439
- DOI: 10.1093/jxb/erv468
Stability of small ubiquitin-like modifier (SUMO) proteases OVERLY TOLERANT TO SALT1 and -2 modulates salicylic acid signalling and SUMO1/2 conjugation in Arabidopsis thaliana
Abstract
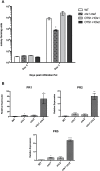
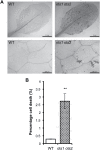
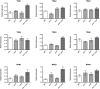
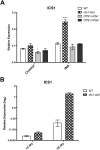
Small ubiquitin-like modifier proteases 1 and 2 (SUMO1/2) have been linked to the regulation of salicylic acid (SA)-mediated defence signalling in Arabidopsis thaliana. In order to define the role of the SUMO proteases OVERLY TOLERANT TO SALT1 and -2 (OTS1/2) in defence and to provide insight into SUMO1/2-mediated regulation of SA signalling, we examined the status of SA-mediated defences in ots1/2 mutants. The ots1 ots2 double mutant displayed enhanced resistance to virulent Pseudomonas syringae and higher levels of SA compared with wild-type (WT) plants. Furthermore, ots1 ots2 mutants exhibited upregulated expression of the SA biosynthesis gene ICS1 in addition to enhanced SA-responsive ICS1 expression beyond that of WT. SA stimulated OTS1/2 degradation and promoted accumulation of SUMO1/2 conjugates. These results indicate that OTS1 and -2 act in a feedback loop in SA signalling and that de novo OTS1/2 synthesis works antagonistically to SA-promoted degradation, adjusting the abundance of OTS1/2 to moderate SA signalling. Accumulation of SUMO1/2 conjugates coincides with SA-promoted OTS degradation and may play a positive role in SA-mediated signalling in addition to its repressive roles reported elsewhere.
Keywords: Arabidopsis thaliana; SUMO protease; SUMOylation.; defence; pathogen; salicylic acid (SA); small ubiquitin-like modifier (SUMO).
© The Author 2015. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures


References
-
- Aftab T, Khan MMA, Teixeira da Silva JA, Idrees M, Naeem M, Moinuddin 2011. Role of salicylic acid in promoting salt stress tolerance and enhanced artemisinin production in Artemisia annua L. Journal of Plant Growth Regulation 30, 425–435.
-
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. 1997. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88, 57–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/D007046/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/D017319/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F005903/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/D017319/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

