FoxP1 orchestration of ASD-relevant signaling pathways in the striatum
- PMID: 26494785
- PMCID: PMC4617974
- DOI: 10.1101/gad.267989.115
FoxP1 orchestration of ASD-relevant signaling pathways in the striatum
Abstract
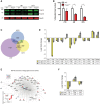
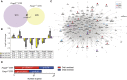
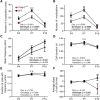
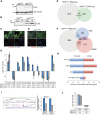
Mutations in the transcription factor Forkhead box p1 (FOXP1) are causative for neurodevelopmental disorders such as autism. However, the function of FOXP1 within the brain remains largely uncharacterized. Here, we identify the gene expression program regulated by FoxP1 in both human neural cells and patient-relevant heterozygous Foxp1 mouse brains. We demonstrate a role for FoxP1 in the transcriptional regulation of autism-related pathways as well as genes involved in neuronal activity. We show that Foxp1 regulates the excitability of striatal medium spiny neurons and that reduction of Foxp1 correlates with defects in ultrasonic vocalizations. Finally, we demonstrate that FoxP1 has an evolutionarily conserved role in regulating pathways involved in striatal neuron identity through gene expression studies in human neural progenitors with altered FOXP1 levels. These data support an integral role for FoxP1 in regulating signaling pathways vulnerable in autism and the specific regulation of striatal pathways important for vocal communication.
Keywords: autism; gene expression; neuronal activity; striatum; ultrasonic vocalizations.
© 2015 Araujo et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


Similar articles
-
Foxp1 in Forebrain Pyramidal Neurons Controls Gene Expression Required for Spatial Learning and Synaptic Plasticity.J Neurosci. 2017 Nov 8;37(45):10917-10931. doi: 10.1523/JNEUROSCI.1005-17.2017. Epub 2017 Oct 4. J Neurosci. 2017. PMID: 28978667 Free PMC article.
-
FoxP1 marks medium spiny neurons from precursors to maturity and is required for their differentiation.Exp Neurol. 2016 Aug;282:9-18. doi: 10.1016/j.expneurol.2016.05.002. Epub 2016 May 3. Exp Neurol. 2016. PMID: 27154297 Free PMC article.
-
Foxp1 expression is essential for sex-specific murine neonatal ultrasonic vocalization.Hum Mol Genet. 2017 Apr 15;26(8):1511-1521. doi: 10.1093/hmg/ddx055. Hum Mol Genet. 2017. PMID: 28204507
-
Autism-like features and FOXP1 syndrome: A scoping review.Brain Dev. 2025 Jun;47(3):104346. doi: 10.1016/j.braindev.2025.104346. Epub 2025 Mar 17. Brain Dev. 2025. PMID: 40101508
-
FOXP1 syndrome: a review of the literature and practice parameters for medical assessment and monitoring.J Neurodev Disord. 2021 Apr 23;13(1):18. doi: 10.1186/s11689-021-09358-1. J Neurodev Disord. 2021. PMID: 33892622 Free PMC article. Review.
Cited by
-
Possible roles of deep cortical neurons and oligodendrocytes in the neural basis of human sociality.Anat Sci Int. 2024 Jan;99(1):34-47. doi: 10.1007/s12565-023-00747-1. Epub 2023 Nov 27. Anat Sci Int. 2024. PMID: 38010534 Free PMC article. Review.
-
The forkhead transcription factor FKH-7/FOXP acts in chemosensory neurons to regulate developmental decision-making.bioRxiv [Preprint]. 2025 Feb 20:2025.02.17.638733. doi: 10.1101/2025.02.17.638733. bioRxiv. 2025. PMID: 40027766 Free PMC article. Preprint.
-
Convergence of spectrums: neuronal gene network states in autism spectrum disorder.Curr Opin Neurobiol. 2019 Dec;59:102-111. doi: 10.1016/j.conb.2019.04.011. Epub 2019 Jun 18. Curr Opin Neurobiol. 2019. PMID: 31220745 Free PMC article. Review.
-
MCRIP1 promotes the expression of lung-surfactant proteins in mice by disrupting CtBP-mediated epigenetic gene silencing.Commun Biol. 2019 Jun 20;2:227. doi: 10.1038/s42003-019-0478-3. eCollection 2019. Commun Biol. 2019. PMID: 31240265 Free PMC article.
-
A MYT1L syndrome mouse model recapitulates patient phenotypes and reveals altered brain development due to disrupted neuronal maturation.Neuron. 2021 Dec 1;109(23):3775-3792.e14. doi: 10.1016/j.neuron.2021.09.009. Epub 2021 Oct 5. Neuron. 2021. PMID: 34614421 Free PMC article.
References
-
- Agmon A, Connors BW. 1991. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41: 365–379. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
