Four-Week Studies of Oral Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitor GSK1278863 for Treatment of Anemia
- PMID: 26494831
- PMCID: PMC4814173
- DOI: 10.1681/ASN.2014111139
Four-Week Studies of Oral Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitor GSK1278863 for Treatment of Anemia
Abstract
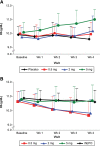
Hypoxia-inducible factor prolyl hydroxylase inhibitors stabilize levels of hypoxia-inducible factor that upregulate transcription of multiple genes associated with the response to hypoxia, including production of erythropoietin. We conducted two phase 2a studies to explore the relationship between the dose of the hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 and hemoglobin response in patients with anemia of CKD (baseline hemoglobin 8.5-11.0 g/dl) not undergoing dialysis and not receiving recombinant human erythropoietin (nondialysis study) and in patients with anemia of CKD (baseline hemoglobin 9.5-12.0 g/dl) on hemodialysis and being treated with stable doses of recombinant human erythropoietin (hemodialysis study). Participants were randomized 1:1:1:1 to a once-daily oral dose of GSK1278863 (0.5 mg, 2 mg, or 5 mg) or control (placebo for the nondialysis study; continuing on recombinant human erythropoietin for the hemodialysis study) for 4 weeks, with a 2-week follow-up. In the nondialysis study, GSK1278863 produced dose-dependent effects on hemoglobin, with the highest dose resulting in a mean increase of 1 g/dl at week 4. In the hemodialysis study, treatment with GSK1278863 in the 5-mg arm maintained mean hemoglobin concentrations after the switch from recombinant human erythropoietin, whereas mean hemoglobin decreased in the lower-dose arms. In both studies, the effects on hemoglobin occurred with elevations in endogenous erythropoietin within the range usually observed in the respective populations and markedly lower than those in the recombinant human erythropoietin control arm in the hemodialysis study, and without clinically significant elevations in plasma vascular endothelial growth factor concentrations. GSK1278863 was generally safe and well tolerated at the doses and duration studied. GSK1278863 may prove an effective alternative for managing anemia of CKD.
Trial registration: ClinicalTrials.gov NCT01587898 NCT01587924.
Keywords: CKD; anemia; erythropoietin.
Copyright © 2016 by the American Society of Nephrology.
Figures
Comment in
-
The Dawning of a New Day in CKD Anemia Care?J Am Soc Nephrol. 2016 Apr;27(4):968-70. doi: 10.1681/ASN.2015091009. Epub 2015 Oct 22. J Am Soc Nephrol. 2016. PMID: 26494832 Free PMC article. No abstract available.
References
-
- Vaziri ND, Zhou XJ: Potential mechanisms of adverse outcomes in trials of anemia correction with erythropoietin in chronic kidney disease. Nephrol Dial Transplant 24: 1082–1088, 2009 - PubMed
-
- Nurko S: Anemia in chronic kidney disease: Causes, diagnosis, treatment. Cleve Clin J Med 73: 289–297, 2006 - PubMed
-
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2: 279–335, 2012
-
- Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713–735, 2014 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

