Roxadustat (FG-4592): Correction of Anemia in Incident Dialysis Patients
- PMID: 26494833
- PMCID: PMC4814189
- DOI: 10.1681/ASN.2015030241
Roxadustat (FG-4592): Correction of Anemia in Incident Dialysis Patients
Abstract
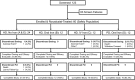
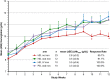
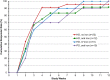
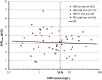
Safety concerns with erythropoietin analogues and intravenous (IV) iron for treatment of anemia in CKD necessitate development of safer therapies. Roxadustat (FG-4592) is an orally bioavailable hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitor that promotes coordinated erythropoiesis through HIF-mediated transcription. We performed an open-label, randomized hemoglobin (Hb) correction study in anemic (Hb≤10.0 g/dl) patients incident to hemodialysis (HD) or peritoneal dialysis (PD). Sixty patients received no iron, oral iron, or IV iron while treated with roxadustat for 12 weeks. Mean±SD baseline Hb was 8.3±1.0 g/dl in enrolled patients. Roxadustat at titrated doses increased mean Hb by ≥2.0 g/dl within 7 weeks regardless of baseline iron repletion status, C-reactive protein level, iron regimen, or dialysis modality. Mean±SEM maximal change in Hb from baseline (ΔHb(max)), the primary endpoint, was 3.1±0.2 g/dl over 12 weeks in efficacy-evaluable patients (n=55). In groups receiving oral or IV iron, ΔHb(max) was similar and larger than in the no-iron group. Hb response (increase in Hb of ≥1.0 g/dl from baseline) was achieved in 96% of efficacy-evaluable patients. Mean serum hepcidin decreased significantly 4 weeks into study: by 80% in HD patients receiving no iron (n=22), 52% in HD and PD patients receiving oral iron (n=21), and 41% in HD patients receiving IV iron (n=9). In summary, roxadustat was well tolerated and corrected anemia in incident HD and PD patients, regardless of baseline iron repletion status or C-reactive protein level and with oral or IV iron supplementation; it also reduced serum hepcidin levels.
Keywords: anemia; chronic kidney disease; dialysis; erythropoietin.
Copyright © 2016 by the American Society of Nephrology.
Figures
Comment in
-
The Dawning of a New Day in CKD Anemia Care?J Am Soc Nephrol. 2016 Apr;27(4):968-70. doi: 10.1681/ASN.2015091009. Epub 2015 Oct 22. J Am Soc Nephrol. 2016. PMID: 26494832 Free PMC article. No abstract available.
References
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 - PubMed
-
- U.S. Renal Data System: 2013 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. Available at http://www.usrds.org/atlas.htm. Accessed October 1, 2014
-
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 - PubMed
-
- KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 50: 471–530, 2007 - PubMed
-
- Arbor Research Collaborative For Health. DOPPS Practice Monitor: 2013 Annual Report of the Dialysis Outcomes and Practice Patterns Study: Hemodialysis Data 1997-2012. Available at http://www.dopps.org/annual report/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

