Evaluation and treatment of malignant ascites secondary to gastric cancer
- PMID: 26494952
- PMCID: PMC4607895
- DOI: 10.3748/wjg.v21.i39.10936
Evaluation and treatment of malignant ascites secondary to gastric cancer
Abstract
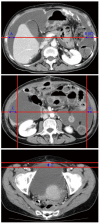
Malignant ascites affects approximately 10% of patients with gastric cancer (GC), and poses significant difficulties for both patients and clinicians. In addition to the dismal general condition of affected patients and the diversity of associated complications such as jaundice and ileus, problems in assessing scattered tumors have hampered the expansion of clinical trials for this condition. However, the accumulation of reported studies is starting to indicate that the weak response to treatment in GC patients with malignant ascites is more relevant to their poor prognosis rather than to the ascites volume at diagnosis. Therefore, precise assessment of initial state of ascites, repetitive evaluation of treatment efficacy, selection of suitable treatment, and swift transition to other treatment options as needed are paramount to maximizing patient benefit. Accurately determining ascites volume is the crucial first step in clinically treating a patient with malignant ascites. Ultrasonography is commonly used to identify the existence of ascites, and several methods have been proposed to estimate ascites volume. Reportedly, the sum of the depth of ascites at five points (named "five-point method") on three panels of computed tomography images is well correlated to the actual ascites volume and/or abdominal girth. This method is already suited to repetitive assessment due to its convenience compared to the conventional volume rendering method. Meanwhile, a new concept, "Clinical Benefit Response in GC (CBR-GC)", was recently introduced to measure the efficacy of chemotherapy for malignant ascites of GC. CBR-GC is a simple and reliable patient-oriented evaluation system based on changes in performance status and ascites, and is expected to become an important clinical endpoint in future clinical trials. The principal of treatment for GC patients with ascites is palliation and prevention of ascites-related symptoms. The treatment options are various, including a standard treatment based on the available guidelines, cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), laparoscopic HIPEC alone, intravenous chemotherapy, intraperitoneal chemotherapy, and molecular targeting therapy. Although each treatment option is valid, further research is imperative to establish the optimal choice for each patient.
Keywords: Ascites; Clinical benefit; Gastric cancer; Paclitaxel; Peritoneal dissemination.
Figures

Similar articles
-
Hyperthermic intraperitoneal chemotherapy plus simultaneous versus staged cytoreductive surgery for gastric cancer with occult peritoneal metastasis.J Surg Oncol. 2015 Jun;111(7):840-7. doi: 10.1002/jso.23889. Epub 2015 Apr 10. J Surg Oncol. 2015. PMID: 25864884
-
[Laparoscopic intraperitoneal hyperthermic perfusion in palliation of malignant ascites. Case report].G Chir. 2009 May;30(5):237-9. G Chir. 2009. PMID: 19505418 Italian.
-
Cell-free and concentrated ascites reinfusion therapy (CART) for management of massive malignant ascites in gastric cancer patients with peritoneal metastasis treated with intravenous and intraperitoneal paclitaxel with oral S-1.Eur J Surg Oncol. 2015 Jul;41(7):875-80. doi: 10.1016/j.ejso.2015.04.013. Epub 2015 May 8. Eur J Surg Oncol. 2015. PMID: 25986856
-
Treatment and prevention of peritoneal carcinomatosis from gastric cancer by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: overview and rationale.Eur J Surg Oncol. 2013 Dec;39(12):1309-16. doi: 10.1016/j.ejso.2013.10.010. Epub 2013 Oct 23. Eur J Surg Oncol. 2013. PMID: 24183797 Review.
-
Looking up: Recent advances in understanding and treating peritoneal carcinomatosis.CA Cancer J Clin. 2015 Jul-Aug;65(4):284-98. doi: 10.3322/caac.21277. Epub 2015 May 4. CA Cancer J Clin. 2015. PMID: 25940594 Review.
Cited by
-
An inguinal hernia revealing an advanced stage gastric cancer in a young patient: A case report.Ann Med Surg (Lond). 2022 Jun 9;79:103974. doi: 10.1016/j.amsu.2022.103974. eCollection 2022 Jul. Ann Med Surg (Lond). 2022. PMID: 35860116 Free PMC article.
-
Relationship between infiltrating lymphocytes in cancerous ascites and dysfunction of Cajal mesenchymal cells in the small intestine.Int J Clin Exp Pathol. 2018 Apr 1;11(4):2201-2213. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31938332 Free PMC article.
-
Gene Expression Profile-Guided Personalized Intraperitoneal Chemotherapy for Gastric Cancer Peritoneal Carcinomatosis.World J Oncol. 2024 Apr;15(2):298-308. doi: 10.14740/wjon1578. Epub 2024 Mar 21. World J Oncol. 2024. PMID: 38545480 Free PMC article.
-
Quantification of ascites based on abdomino-pelvic computed tomography scans for predicting the in-hospital mortality of liver cirrhosis.Exp Ther Med. 2017 Dec;14(6):5733-5742. doi: 10.3892/etm.2017.5321. Epub 2017 Oct 17. Exp Ther Med. 2017. PMID: 29285115 Free PMC article.
-
Neoadjuvant intraperitoneal chemotherapy for advanced stage gastric cancer (Review).Exp Ther Med. 2021 Nov;22(5):1314. doi: 10.3892/etm.2021.10749. Epub 2021 Sep 17. Exp Ther Med. 2021. PMID: 34630668 Free PMC article. Review.
References
-
- Imamoto H, Oba K, Sakamoto J, Iishi H, Narahara H, Yumiba T, Morimoto T, Nakamura M, Oriuchi N, Kakutani C, et al. Assessing clinical benefit response in the treatment of gastric malignant ascites with non-measurable lesions: a multicenter phase II trial of paclitaxel for malignant ascites secondary to advanced/recurrent gastric cancer. Gastric Cancer. 2011;14:81–90. - PubMed
-
- Iwasa S, Nakajima TE, Nakamura K, Takashima A, Kato K, Hamaguchi T, Yamada Y, Shimada Y. First-line fluorouracil-based chemotherapy for patients with severe peritoneal disseminated gastric cancer. Gastric Cancer. 2012;15:21–26. - PubMed
-
- Oriuchi N, Nakajima T, Mochiki E, Takeyoshi I, Kanuma T, Endo K, Sakamoto J. A new, accurate and conventional five-point method for quantitative evaluation of ascites using plain computed tomography in cancer patients. Jpn J Clin Oncol. 2005;35:386–390. - PubMed
-
- Barni S, Cabiddu M, Ghilardi M, Petrelli F. A novel perspective for an orphan problem: old and new drugs for the medical management of malignant ascites. Crit Rev Oncol Hematol. 2011;79:144–153. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

