Suppression of Propionibacterium acnes-Induced Dermatitis by a Traditional Japanese Medicine, Jumihaidokuto, Modifying Macrophage Functions
- PMID: 26495013
- PMCID: PMC4606168
- DOI: 10.1155/2015/439258
Suppression of Propionibacterium acnes-Induced Dermatitis by a Traditional Japanese Medicine, Jumihaidokuto, Modifying Macrophage Functions
Abstract
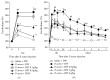
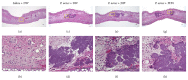
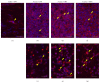
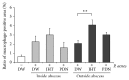
Purpose. Macrophages serve as sweepers of microbes and inflammation-derived wastes and regulators of inflammation. Some traditional Japanese medicines are reported to have adjuvant effects by modifying macrophages. Our aim was to characterize the actions of jumihaidokuto (JHT) for treatment of skin inflammations including acne vulgaris, in which Propionibacterium acnes has pathogenic roles. Methods. Dermatitis was induced in rat ears by intradermal injection of P. acnes. JHT or prednisolone (PDN) was given orally, and ear thickness and histology were evaluated. The effects of constituents and metabolites of JHT on monocytes were tested by cell-based assays using the human monocytic THP-1 cell. Results. JHT and PDN suppressed the ear thickness induced by P. acnes injection. Histological examinations revealed that JHT, but not PDN, promoted macrophage accumulation at 24 h after the injection. PDN suppressed the macrophage chemokine MCP-1 in the inflamed ears, while JHT did not affect it. The JHT constituents liquiritigenin and isoliquiritin increased expression of CD86 (type-1 macrophage marker) and CD192 (MCP-1 receptor) and enhanced phagocytosis by THP-1. Conclusions. JHT suppressed dermatitis, probably by enhancing type-1 macrophage functions, with an action different from PDN. JHT may be a beneficial drug in treatment of skin inflammation induced by P. acnes.
Figures

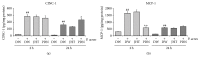
Similar articles
-
Plasma Pharmacokinetics of Polyphenols in a Traditional Japanese Medicine, Jumihaidokuto, Which Suppresses Propionibacterium acnes-Induced Dermatitis in Rats.Molecules. 2015 Sep 30;20(10):18031-46. doi: 10.3390/molecules201018031. Molecules. 2015. PMID: 26437394 Free PMC article.
-
Relationship between Propionibacterium acnes biotypes and Jumi-haidoku-to.J Dermatol. 2000 Oct;27(10):635-8. doi: 10.1111/j.1346-8138.2000.tb02244.x. J Dermatol. 2000. PMID: 11092266
-
Effect of lipase activities of Propionibacterium granulosum and Propionibacterium acnes.Drugs Exp Clin Res. 2001;27(5-6):161-4. Drugs Exp Clin Res. 2001. PMID: 11951573
-
Propionibacterium acnes in the pathogenesis and immunotherapy of acne vulgaris.Curr Drug Metab. 2015;16(4):245-54. doi: 10.2174/1389200216666150812124801. Curr Drug Metab. 2015. PMID: 26264195 Review.
-
Propionibacterium (Cutibacterium) acnes Bacteriophage Therapy in Acne: Current Evidence and Future Perspectives.Dermatol Ther (Heidelb). 2019 Mar;9(1):19-31. doi: 10.1007/s13555-018-0275-9. Epub 2018 Dec 11. Dermatol Ther (Heidelb). 2019. PMID: 30539425 Free PMC article. Review.
Cited by
-
Research progress on the role of macrophages in acne and regulation by natural plant products.Front Immunol. 2024 Apr 26;15:1383263. doi: 10.3389/fimmu.2024.1383263. eCollection 2024. Front Immunol. 2024. PMID: 38736879 Free PMC article. Review.
-
Inhibition of Human Kallikrein 5 Protease by Triterpenoids from Natural Sources.Molecules. 2017 Oct 27;22(11):1829. doi: 10.3390/molecules22111829. Molecules. 2017. PMID: 29077044 Free PMC article.
-
Macrophages in acne vulgaris: mediating phagocytosis, inflammation, scar formation, and therapeutic implications.Front Immunol. 2024 Mar 14;15:1355455. doi: 10.3389/fimmu.2024.1355455. eCollection 2024. Front Immunol. 2024. PMID: 38550588 Free PMC article. Review.
-
Effect of β-agonist on the dexamethasone-induced expression of aromatase by the human monocyte cells.Endocr Connect. 2017 Feb;6(2):82-88. doi: 10.1530/EC-16-0099. Epub 2017 Jan 26. Endocr Connect. 2017. PMID: 28126832 Free PMC article.
-
Glucuronides of phytoestrogen flavonoid enhance macrophage function via conversion to aglycones by β-glucuronidase in macrophages.Immun Inflamm Dis. 2017 Sep;5(3):265-279. doi: 10.1002/iid3.163. Epub 2017 May 8. Immun Inflamm Dis. 2017. PMID: 28480538 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

