Propofol requirement for induction of unconsciousness is reduced in patients with Parkinson's disease: a case control study
- PMID: 26495319
- PMCID: PMC4606158
- DOI: 10.1155/2015/953729
Propofol requirement for induction of unconsciousness is reduced in patients with Parkinson's disease: a case control study
Abstract
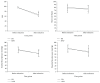
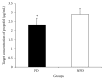
Parkinson's disease (PD) is the second most prevalent neurodegenerative disease, but whether the neurodegenerative process influences the pharmacodynamics of propofol remains unclear. We aimed to evaluate the effect of PD on pharmacodynamics of propofol. A total of 31 PD patients undergoing surgical treatment (PD group) and 31 pair-controlled non-PD patients undergoing intracranial surgery (NPD group) were recruited to investigate the propofol requirement for unconsciousness induction. Unconsciousness was induced in all patients with target-controlled infusion of propofol. The propofol concentration at which unconsciousness was induced was compared between the two groups. EC50 and EC95 were calculated as well. Demographic data, bispectral index, and hemodynamic values were comparable between PD and NPD groups. The mean target concentration of propofol when unconsciousness was achieved was 2.32 ± 0.38 μg/mL in PD group, which was significantly lower than that in NPD group (2.90 ± 0.35 μg/mL). The EC50 was 2.05 μg/mL (95% CI: 1.85-2.19 μg/mL) in PD group, much lower than the 2.72 μg/mL (95% CI: 2.53-2.88 μg/mL) in NPD group. In conclusion, the effective propofol concentration needed for induction of unconsciousness in 50% of patients is reduced in PD patients. (This trial is registered with NCT01998204.).
Figures
Similar articles
-
Comparison of Half-Effective Concentration of Propofol in Patients with Parkinson's Disease and Non-Parkinson's Disease.Clin Interv Aging. 2023 Feb 28;18:307-315. doi: 10.2147/CIA.S380416. eCollection 2023. Clin Interv Aging. 2023. PMID: 36879829 Free PMC article.
-
Remifentanil requirement for i-gel insertion is reduced in male patients with Parkinson's disease undergoing deep brain stimulator implantation: an up-and-down sequential allocation trial.BMC Anesthesiol. 2022 Jun 24;22(1):197. doi: 10.1186/s12871-022-01735-0. BMC Anesthesiol. 2022. PMID: 35751029 Free PMC article. Clinical Trial.
-
Remifentanil Requirement for Inhibiting Responses to Tracheal Intubation and Skin Incision Is Reduced in Patients With Parkinson's Disease Undergoing Deep Brain Stimulator Implantation.J Neurosurg Anesthesiol. 2016 Oct;28(4):303-8. doi: 10.1097/ANA.0000000000000229. J Neurosurg Anesthesiol. 2016. PMID: 26368663
-
[Comparison of unconsciousness prediction probability with approximate entropy and bispectral index during sedation].Nan Fang Yi Ke Da Xue Xue Bao. 2006 Mar;26(3):287-9. Nan Fang Yi Ke Da Xue Xue Bao. 2006. PMID: 16546728 Clinical Trial. Chinese.
-
Bispectral index as a guide for titration of propofol during procedural sedation among children.Pediatrics. 2005 Jun;115(6):1666-74. doi: 10.1542/peds.2004-1979. Pediatrics. 2005. PMID: 15930231 Review.
Cited by
-
Minimum Alveolar Concentration-Awake of Sevoflurane is Decreased in Patients with Parkinson's Disease: An Up-and-Down Sequential Allocation Trial.Clin Interv Aging. 2021 Jan 15;16:129-137. doi: 10.2147/CIA.S291656. eCollection 2021. Clin Interv Aging. 2021. PMID: 33488069 Free PMC article. Clinical Trial.
-
Propofol-induced loss of consciousness is associated with a decrease in thalamocortical connectivity in humans.Brain. 2019 Aug 1;142(8):2288-2302. doi: 10.1093/brain/awz169. Brain. 2019. PMID: 31236577 Free PMC article.
-
Globus pallidus externus drives increase in network-wide alpha power with propofol-induced loss-of-consciousness in humans.Cereb Cortex. 2024 Jun 4;34(6):bhae243. doi: 10.1093/cercor/bhae243. Cereb Cortex. 2024. PMID: 38850214 Free PMC article.
-
Comparison of Half-Effective Concentration of Propofol in Patients with Parkinson's Disease and Non-Parkinson's Disease.Clin Interv Aging. 2023 Feb 28;18:307-315. doi: 10.2147/CIA.S380416. eCollection 2023. Clin Interv Aging. 2023. PMID: 36879829 Free PMC article.
-
Remifentanil requirement for i-gel insertion is reduced in male patients with Parkinson's disease undergoing deep brain stimulator implantation: an up-and-down sequential allocation trial.BMC Anesthesiol. 2022 Jun 24;22(1):197. doi: 10.1186/s12871-022-01735-0. BMC Anesthesiol. 2022. PMID: 35751029 Free PMC article. Clinical Trial.
References
-
- de Lau L. M. L., Giesbergen P. C. L. M., de Rijk M. C., Hofman A., Koudstaal P. J., Breteler M. M. B. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam study. Neurology. 2004;63(7):1240–1244. doi: 10.1212/01.wnl.0000140706.52798.be. - DOI - PubMed
-
- Benabid A. L., Pollak P., Louveau A., Henry S., de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Applied Neurophysiology. 1987;50:344–346. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

