Competitive inhibition can linearize dose-response and generate a linear rectifier
- PMID: 26495436
- PMCID: PMC4610030
- DOI: 10.1016/j.cels.2015.09.001
Competitive inhibition can linearize dose-response and generate a linear rectifier
Abstract
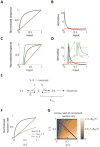
Many biological responses require a dynamic range that is larger than standard bi-molecular interactions allow, yet the also ability to remain off at low input. Here we mathematically show that an enzyme reaction system involving a combination of competitive inhibition, conservation of the total level of substrate and inhibitor, and positive feedback can behave like a linear rectifier-that is, a network motif with an input-output relationship that is linearly sensitive to substrate above a threshold but unresponsive below the threshold. We propose that the evolutionarily conserved yeast SAGA histone acetylation complex may possess the proper physiological response characteristics and molecular interactions needed to perform as a linear rectifier, and we suggest potential experiments to test this hypothesis. One implication of this work is that linear responses and linear rectifiers might be easier to evolve or synthetically construct than is currently appreciated.
Keywords: Competitive inhibition; Enzyme kinetics; Linear rectifiers; SAGA; Transcriptional regulation.
Figures
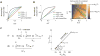


Similar articles
-
RF rectifiers for EM power harvesting in a Deep Brain Stimulating device.Australas Phys Eng Sci Med. 2015 Mar;38(1):157-72. doi: 10.1007/s13246-015-0328-7. Epub 2015 Jan 20. Australas Phys Eng Sci Med. 2015. PMID: 25600671
-
Chd1 chromodomain links histone H3 methylation with SAGA- and SLIK-dependent acetylation.Nature. 2005 Jan 27;433(7024):434-8. doi: 10.1038/nature03242. Epub 2005 Jan 12. Nature. 2005. PMID: 15647753
-
Catalytic properties and kinetic mechanism of human recombinant Lys-9 histone H3 methyltransferase SUV39H1: participation of the chromodomain in enzymatic catalysis.Biochemistry. 2006 Mar 14;45(10):3272-84. doi: 10.1021/bi051997r. Biochemistry. 2006. PMID: 16519522
-
Multi-tasking on chromatin with the SAGA coactivator complexes.Mutat Res. 2007 May 1;618(1-2):135-48. doi: 10.1016/j.mrfmmm.2006.09.008. Epub 2007 Jan 21. Mutat Res. 2007. PMID: 17337012 Free PMC article. Review.
-
Recruitment of chromatin remodelling factors during gene activation via the glucocorticoid receptor N-terminal domain.Biochem Soc Trans. 2000;28(4):410-4. Biochem Soc Trans. 2000. PMID: 10961930 Review.
Cited by
-
Roles of WNK4 and SPAK in K+-mediated dephosphorylation of the NaCl cotransporter.Am J Physiol Renal Physiol. 2021 May 1;320(5):F719-F733. doi: 10.1152/ajprenal.00459.2020. Epub 2021 Mar 15. Am J Physiol Renal Physiol. 2021. PMID: 33719576 Free PMC article.
-
In Vitro Monitoring of the Mitochondrial Beta-Oxidation Flux of Palmitic Acid and Investigation of Its Pharmacological Alteration by Therapeutics.Eur J Drug Metab Pharmacokinet. 2018 Dec;43(6):675-684. doi: 10.1007/s13318-018-0479-5. Eur J Drug Metab Pharmacokinet. 2018. PMID: 29725943
-
Self-replenishment cycles generate a threshold response.Sci Rep. 2019 Nov 20;9(1):17139. doi: 10.1038/s41598-019-53589-1. Sci Rep. 2019. PMID: 31748624 Free PMC article.
-
Chemoproteomics Yields a Selective Molecular Host for Acetyl-CoA.J Am Chem Soc. 2023 Aug 2;145(30):16899-16905. doi: 10.1021/jacs.3c05489. Epub 2023 Jul 24. J Am Chem Soc. 2023. PMID: 37486078 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

