Side-effects of SSRIs disrupt multimodal treatment for pediatric OCD in a randomized-controlled trial
- PMID: 26495770
- PMCID: PMC4653063
- DOI: 10.1016/j.jpsychires.2015.10.006
Side-effects of SSRIs disrupt multimodal treatment for pediatric OCD in a randomized-controlled trial
Erratum in
- J Psychiatr Res. 2016 Mar;74:94. Goodman, Wayne K [added]
Abstract
Objective: Activation Syndrome (AS) is a side-effect of antidepressants consisting of irritability, mania, self-harm, akathisia, and disinhibition. The current study was conducted to analyze how AS may hinder treatment outcome for multimodal treatment for children and adolescents with Obsessive-Compulsive Disorder.
Methods: Fifty-six children or adolescents were recruited at two treatment sites in a double-blind randomized-controlled trial where participants received Cognitive-Behavioral Therapy and were randomized to slow titration of sertraline, regular titration of sertraline or placebo.
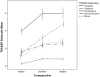
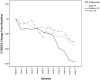
Results: Using a recently developed measure of AS, results suggested that higher average levels of irritability, akathisia, and disinhibition significantly interfered with treatment response and explained 18% of the variance in obsessive-compulsive symptoms during treatment. Interestingly, only session-to-session increases in irritability resulted in a session-to-session increase in obsessive-compulsive symptoms. The observed results were unchanged with the addition of SSRI dosage as a covariate.
Conclusions: Results provide empirical support for the proposed hypothesis that AS may hinder multimodal treatment outcome for pediatric OCD. These findings suggest that dosage changes due to AS do not explain why those with higher AS had worse multimodal outcome. Other possible mechanisms explaining this observed disruption are proposed, including how AS may interfere with Cognitive-Behavioral Therapy.
Trial registration: ClinicalTrials.gov NCT00382291.
Keywords: Activation syndrome; Children; Cognitive-behavioral therapy; Obsessive-Compulsive Disorder; Selective serotonin reuptake inhibitors; Side-effects.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial.JAMA. 2004 Oct 27;292(16):1969-76. doi: 10.1001/jama.292.16.1969. JAMA. 2004. PMID: 15507582 Clinical Trial.
-
Randomized, placebo-controlled trial of cognitive-behavioral therapy alone or combined with sertraline in the treatment of pediatric obsessive-compulsive disorder.Behav Res Ther. 2013 Dec;51(12):823-9. doi: 10.1016/j.brat.2013.09.007. Epub 2013 Oct 10. Behav Res Ther. 2013. PMID: 24184429 Free PMC article. Clinical Trial.
-
Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis.J Clin Psychiatry. 2016 May;77(5):e605-11. doi: 10.4088/JCP.14r09758. J Clin Psychiatry. 2016. PMID: 27249090
-
Sertraline in the treatment of obsessive compulsive disorder: two double-blind, placebo-controlled studies.Int Clin Psychopharmacol. 1992 Oct;7 Suppl 2:37-41. doi: 10.1097/00004850-199210002-00007. Int Clin Psychopharmacol. 1992. PMID: 1484177 Review.
-
[A Review and Recommendations of Evidence-Based Treatments for Pediatric Obsessive-Compulsive Disorder].Laeknabladid. 2016 Apr;102(4):179-85. doi: 10.17992/lbl.2016.04.75. Laeknabladid. 2016. PMID: 27197125 Review. Icelandic.
Cited by
-
Pharmacogenetically Guided Escitalopram Treatment for Pediatric Anxiety Disorders: Protocol for a Double-Blind Randomized Trial.J Pers Med. 2021 Nov 12;11(11):1188. doi: 10.3390/jpm11111188. J Pers Med. 2021. PMID: 34834540 Free PMC article.
-
Curcumin Alters Neural Plasticity and Viability of Intact Hippocampal Circuits and Attenuates Behavioral Despair and COX-2 Expression in Chronically Stressed Rats.Mediators Inflamm. 2017;2017:6280925. doi: 10.1155/2017/6280925. Epub 2017 Jan 11. Mediators Inflamm. 2017. PMID: 28167853 Free PMC article.
-
Antidepressant-Induced Activation in Children and Adolescents: Risk, Recognition and Management.Curr Probl Pediatr Adolesc Health Care. 2018 Feb;48(2):50-62. doi: 10.1016/j.cppeds.2017.12.001. Epub 2018 Jan 19. Curr Probl Pediatr Adolesc Health Care. 2018. PMID: 29358037 Free PMC article. Review.
-
Anxiolytic-like effects of paeoniflorin in an animal model of post traumatic stress disorder.Metab Brain Dis. 2018 Aug;33(4):1175-1185. doi: 10.1007/s11011-018-0216-4. Epub 2018 Apr 10. Metab Brain Dis. 2018. PMID: 29633071
-
Medicinal plants and plant-based traditional medicine: Alternative treatments for depression and their potential mechanisms of action.Heliyon. 2024 Oct 5;10(20):e38986. doi: 10.1016/j.heliyon.2024.e38986. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39640650 Free PMC article. Review.
References
-
- Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord. 2000;58:19–36. - PubMed
-
- Antony MM, Purdon C, Summerfeldt LJ, editors. Psychological Treatments of Obsessive Compulsive Disorder: Fundamentals and Beyond. American Psychological Association; Washington DC: 2007. pp. 9–29.
-
- Arumugham SS, Reddy JY. Augmentation strategies in obsessive-compulsive disorder. Expert Rev Neurother. 2013;13:187–202. - PubMed
-
- Beasley CM, Jr, Sayler ME, Bosomworth JC, Wernicke JF. High-dose fluoxetine: efficacy and activating-sedating effects in agitated and retarded depression. J Clin Psychopharmacol. 1991;11:166–174. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

