Natural Diels-Alderases: Elusive and Irresistable
- PMID: 26495876
- PMCID: PMC4806863
- DOI: 10.1021/acs.joc.5b01951
Natural Diels-Alderases: Elusive and Irresistable
Abstract
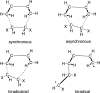
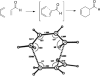
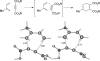
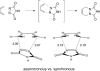
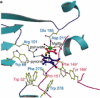
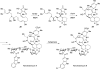
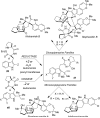
Eight examples of biosynthetic pathways wherein a natural enzyme has been identified and claimed to function as a catalyst for the [4 + 2] cycloaddition reaction, namely, Diels-Alderases, are briefly reviewed. These are discussed in the context of the mechanistic challenges associated with the technical difficulty of proving that the net formal [4 + 2] cycloaddition under study indeed proceeds through a synchronous mechanism and that the putative biosynthetic enzyme deploys the pericyclic transition state required for a Diels-Alder cycloaddition reaction.
Figures
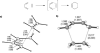
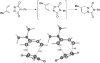
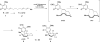

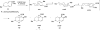
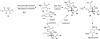

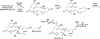
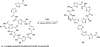



References
-
- Laschat S. Angew. Chem. Int. Ed. 1996;35:289–291.
- Stocking EM, Williams RM. Angew. Chem. Int. Ed. 2003;42:3078–3115. - PubMed
-
- Kelly WL. Org. Biomol. Chem. 2008;6:483–4493. - PubMed
- Oikawa H. Bull. Chem. Soc. Jpn. 2005;78:537–554.
-
- Sauer J, Sustmann R. Angew. Chem. Int. Ed. 1980;19:779–807.
-
- Houk KN. J. Am. Chem. Soc. 1973;95:4092–4094.
- Houk KN, Strozier R. J. Am. Chem. Soc. 1973;94:4094–4096.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

