Deficiency or inhibition of lysophosphatidic acid receptor 1 protects against hyperoxia-induced lung injury in neonatal rats
- PMID: 26495902
- PMCID: PMC4738067
- DOI: 10.1111/apha.12622
Deficiency or inhibition of lysophosphatidic acid receptor 1 protects against hyperoxia-induced lung injury in neonatal rats
Abstract
Aim: Blocking of lysophosphatidic acid (LPA) receptor (LPAR) 1 may be a novel therapeutic option for bronchopulmonary dysplasia (BPD) by preventing the LPAR1-mediated adverse effects of its ligand (LPA), consisting of lung inflammation, pulmonary arterial hypertension (PAH) and fibrosis.
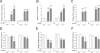
Methods: In Wistar rats with experimental BPD, induced by continuous exposure to 100% oxygen for 10 days, we determined the beneficial effects of LPAR1 deficiency in neonatal rats with a missense mutation in cytoplasmic helix 8 of LPAR1 and of LPAR1 and -3 blocking with Ki16425. Parameters investigated included survival, lung and heart histopathology, fibrin and collagen deposition, vascular leakage and differential mRNA expression in the lungs of key genes involved in LPA signalling and BPD pathogenesis.
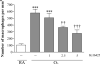
Results: LPAR1-mutant rats were protected against experimental BPD and mortality with reduced alveolar septal thickness, lung inflammation (reduced influx of macrophages and neutrophils, and CINC1 expression) and collagen III deposition. However, LPAR1-mutant rats were not protected against alveolar enlargement, increased medial wall thickness of small arterioles, fibrin deposition and vascular alveolar leakage. Treatment of experimental BPD with Ki16425 confirmed the data observed in LPAR1-mutant rats, but did not reduce the pulmonary influx of neutrophils, CINC1 expression and mortality in rats with experimental BPD. In addition, Ki16425 treatment protected against PAH and right ventricular hypertrophy.
Conclusion: LPAR1 deficiency attenuates pulmonary injury by reducing pulmonary inflammation and fibrosis, thereby reducing mortality, but does not affect alveolar and vascular development and, unlike Ki16425 treatment, does not prevent PAH in neonatal rats with experimental BPD.
Keywords: bronchopulmonary dysplasia; fibrosis; lung inflammation; lysophosphatidic acid receptor; right ventricular hypertrophy.
© 2015 Scandinavian Physiological Society. Published by John Wiley & Sons Ltd.
Figures


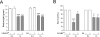
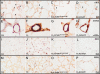
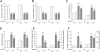

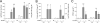
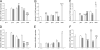


Similar articles
-
Angiotensin II type 2 receptor ligand PD123319 attenuates hyperoxia-induced lung and heart injury at a low dose in newborn rats.Am J Physiol Lung Cell Mol Physiol. 2014 Aug 1;307(3):L261-72. doi: 10.1152/ajplung.00345.2013. Epub 2014 Jun 20. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24951776 Free PMC article.
-
Metformin attenuates hyperoxia-induced lung injury in neonatal rats by reducing the inflammatory response.Am J Physiol Lung Cell Mol Physiol. 2015 Aug 1;309(3):L262-70. doi: 10.1152/ajplung.00389.2014. Epub 2015 Jun 5. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26047641 Free PMC article.
-
Agonists of MAS oncogene and angiotensin II type 2 receptors attenuate cardiopulmonary disease in rats with neonatal hyperoxia-induced lung injury.Am J Physiol Lung Cell Mol Physiol. 2013 Sep;305(5):L341-51. doi: 10.1152/ajplung.00360.2012. Epub 2013 Jun 28. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23812633 Free PMC article.
-
CTGF: A potential therapeutic target for Bronchopulmonary dysplasia.Eur J Pharmacol. 2019 Oct 5;860:172588. doi: 10.1016/j.ejphar.2019.172588. Epub 2019 Aug 1. Eur J Pharmacol. 2019. PMID: 31377154 Review.
-
Inhibition of LPA-LPAR1 and VEGF-VEGFR2 Signaling in IPF Treatment.Drug Des Devel Ther. 2023 Sep 2;17:2679-2690. doi: 10.2147/DDDT.S415453. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37680863 Free PMC article. Review.
Cited by
-
Lysophospholipids in Lung Inflammatory Diseases.Adv Exp Med Biol. 2021;1303:373-391. doi: 10.1007/978-3-030-63046-1_20. Adv Exp Med Biol. 2021. PMID: 33788203 Free PMC article.
-
Emerging Roles of Lysophosphatidic Acid in Macrophages and Inflammatory Diseases.Int J Mol Sci. 2023 Aug 7;24(15):12524. doi: 10.3390/ijms241512524. Int J Mol Sci. 2023. PMID: 37569902 Free PMC article. Review.
-
Expression profiling of genes regulated by sphingosine kinase1 signaling in a murine model of hyperoxia induced neonatal bronchopulmonary dysplasia.BMC Genomics. 2017 Aug 29;18(1):664. doi: 10.1186/s12864-017-4048-0. BMC Genomics. 2017. PMID: 28851267 Free PMC article.
-
Overexpression of AGT promotes bronchopulmonary dysplasis via the JAK/STAT signal pathway.Oncotarget. 2017 Oct 10;8(56):96079-96088. doi: 10.18632/oncotarget.21712. eCollection 2017 Nov 10. Oncotarget. 2017. PMID: 29221188 Free PMC article.
-
Aberrant cGMP signaling persists during recovery in mice with oxygen-induced pulmonary hypertension.PLoS One. 2017 Aug 9;12(8):e0180957. doi: 10.1371/journal.pone.0180957. eCollection 2017. PLoS One. 2017. PMID: 28792962 Free PMC article.
References
-
- Abman SH. Role of endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Annu Rev Med. 2009;60:13–23. - PubMed
-
- Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. - PubMed
-
- Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

