Immunologic Control of Mus musculus Papillomavirus Type 1
- PMID: 26495972
- PMCID: PMC4619818
- DOI: 10.1371/journal.ppat.1005243
Immunologic Control of Mus musculus Papillomavirus Type 1
Abstract
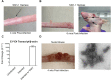
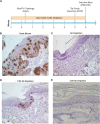
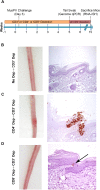
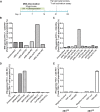
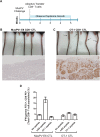
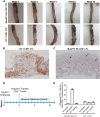
Persistent papillomas developed in ~10% of out-bred immune-competent SKH-1 mice following MusPV1 challenge of their tail, and in a similar fraction the papillomas were transient, suggesting potential as a model. However, papillomas only occurred in BALB/c or C57BL/6 mice depleted of T cells with anti-CD3 antibody, and they completely regressed within 8 weeks after depletion was stopped. Neither CD4+ nor CD8+ T cell depletion alone in BALB/c or C57BL/6 mice was sufficient to permit visible papilloma formation. However, low levels of MusPV1 were sporadically detected by either genomic DNA-specific PCR analysis of local skin swabs or in situ hybridization of the challenge site with an E6/E7 probe. After switching to CD3+ T cell depletion, papillomas appeared upon 14/15 of mice that had been CD4+ T cell depleted throughout the challenge phase, 1/15 of CD8+ T cell depleted mice, and none in mice without any prior T cell depletion. Both control animals and those depleted with CD8-specific antibody generated MusPV1 L1 capsid-specific antibodies, but not those depleted with CD4-specific antibody prior to T cell depletion with CD3 antibody. Thus, normal BALB/c or C57BL/6 mice eliminate the challenge dose, whereas infection is suppressed but not completely cleared if their CD4 or CD8 T cells are depleted, and recrudescence of MusPV1 is much greater in the former following treatment with CD3 antibody, possibly reflecting their failure to generate capsid antibody. Systemic vaccination of C57BL/6 mice with DNA vectors expressing MusPV1 E6 or E7 fused to calreticulin elicits potent CD8 T cell responses and these immunodominant CD8 T cell epitopes were mapped. Adoptive transfer of a MusPV1 E6-specific CD8+ T cell line controlled established MusPV1 infection and papilloma in RAG1-knockout mice. These findings suggest the potential of immunotherapy for HPV-related disease and the importance of host immunogenetics in the outcome of infection.
Conflict of interest statement
We have read the journal's policy and have the following competing interests. Yung-Nien Chang is an employee of Papivax Biotech Inc. and holds stock options, and is a member of Papivax LLC. Richard Roden is an inventor of patents (20090047301 Papillomavirus L2 N-Terminal Peptides for the Induction of Broadly Cross-Neutralizing Antibodies and 20100297144 MULTITYPE HPV PEPTIDE COMPOSITIONS AND METHODS FOR TREATMENT OR PREVENTION OF HUMAN PAPILLOMAVIRUS INFECTION) licensed to Shantha Biotechnics Ltd., GlaxoSmithKline, PaxVax, Inc. and Acambis, Inc. Richard Roden has received research funding from Sanofi Pasteur, Shantha Biotechnic and GlaxoSmithKline, is a member of Papivax LLC, has Papivax Biotech Inc. stock options and is a member of Papivax Biotech Inc.'s Scientific Advisory Board. Papivax Biotech Inc. has licensed technology developed by Chien-fu Hung (7,342,002 Molecular vaccine linking an endoplasmic chaperone polypeptide to an antigen). These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. This does not alter our adherence to all the PLoS ONE policies on sharing data and materials.
Figures

Similar articles
-
Spontaneous and Vaccine-Induced Clearance of Mus Musculus Papillomavirus 1 Infection.J Virol. 2017 Jul 12;91(15):e00699-17. doi: 10.1128/JVI.00699-17. Print 2017 Aug 1. J Virol. 2017. PMID: 28515303 Free PMC article.
-
Strain-specific properties and T cells regulate the susceptibility to papilloma induction by Mus musculus papillomavirus 1.PLoS Pathog. 2014 Aug 14;10(8):e1004314. doi: 10.1371/journal.ppat.1004314. eCollection 2014 Aug. PLoS Pathog. 2014. PMID: 25121947 Free PMC article.
-
CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection.J Virol. 1994 Dec;68(12):8056-63. doi: 10.1128/JVI.68.12.8056-8063.1994. J Virol. 1994. PMID: 7966595 Free PMC article.
-
The role of T-cell subsets in the response to anti-CD3 monoclonal antibodies.Clin Immunol Immunopathol. 1992 Dec;65(3):234-41. doi: 10.1016/0090-1229(92)90152-e. Clin Immunol Immunopathol. 1992. PMID: 1360341
-
CD4+ TH1 cells generated by Ii-PADRE DNA at prime phase are important to induce effectors and memory CD8+ T cells.J Immunother. 2010 Jun;33(5):510-22. doi: 10.1097/CJI.0b013e3181d75cef. J Immunother. 2010. PMID: 20463596
Cited by
-
Recent advances in preclinical model systems for papillomaviruses.Virus Res. 2017 Mar 2;231:108-118. doi: 10.1016/j.virusres.2016.12.004. Epub 2016 Dec 9. Virus Res. 2017. PMID: 27956145 Free PMC article. Review.
-
Absence of γ-Chain in Keratinocytes Alters Chemokine Secretion, Resulting in Reduced Immune Cell Recruitment.J Invest Dermatol. 2017 Oct;137(10):2120-2130. doi: 10.1016/j.jid.2017.05.024. Epub 2017 Jun 17. J Invest Dermatol. 2017. PMID: 28634034 Free PMC article.
-
HPV and the Risk of HIV Acquisition in Women.Front Cell Infect Microbiol. 2022 Feb 10;12:814948. doi: 10.3389/fcimb.2022.814948. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35223546 Free PMC article. Review.
-
Mus musculus Papillomavirus 1: a New Frontier in Animal Models of Papillomavirus Pathogenesis.J Virol. 2020 Apr 16;94(9):e00002-20. doi: 10.1128/JVI.00002-20. Print 2020 Apr 16. J Virol. 2020. PMID: 32051276 Free PMC article. Review.
-
Characterization of HPV18 E6-specific T cell responses and establishment of HPV18 E6-expressing tumor model.Vaccine. 2017 Jul 5;35(31):3850-3858. doi: 10.1016/j.vaccine.2017.05.081. Epub 2017 Jun 7. Vaccine. 2017. PMID: 28599791 Free PMC article.
References
-
- zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2: 342–350. - PubMed
-
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H (2004) Classification of papillomaviruses. Virology 324: 17–27. - PubMed
-
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, et al. (2009) A review of human carcinogens—Part B: biological agents. Lancet Oncol 10: 321–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

