Relationship between Humoral Immune Responses against HPV16, HPV18, HPV31 and HPV45 in 12-15 Year Old Girls Receiving Cervarix® or Gardasil® Vaccine
- PMID: 26495976
- PMCID: PMC4619723
- DOI: 10.1371/journal.pone.0140926
Relationship between Humoral Immune Responses against HPV16, HPV18, HPV31 and HPV45 in 12-15 Year Old Girls Receiving Cervarix® or Gardasil® Vaccine
Abstract
Background: Human papillomavirus (HPV) vaccines confer protection against the oncogenic genotypes HPV16 and HPV18 through the generation of type-specific neutralizing antibodies raised against virus-like particles (VLP) representing these genotypes. The vaccines also confer a degree of cross-protection against HPV31 and HPV45, which are genetically-related to the vaccine types HPV16 and HPV18, respectively, although the mechanism is less certain. There are a number of humoral immune measures that have been examined in relation to the HPV vaccines, including VLP binding, pseudovirus neutralization and the enumeration of memory B cells. While the specificity of responses generated against the vaccine genotypes are fairly well studied, the relationship between these measures in relation to non-vaccine genotypes is less certain.
Methods: We carried out a comparative study of these immune measures against vaccine and non-vaccine genotypes using samples collected from 12-15 year old girls following immunization with three doses of either Cervarix® or Gardasil® HPV vaccine.
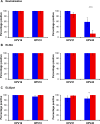
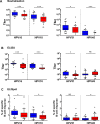
Results: The relationship between neutralizing and binding antibody titers and HPV-specific memory B cell levels for the vaccine genotypes, HPV16 and HPV18, were very good. The proportion of responders approached 100% for both vaccines while the magnitude of these responses induced by Cervarix® were generally higher than those following Gardasil® immunization. A similar pattern was found for the non-vaccine genotype HPV31, albeit at a lower magnitude compared to its genetically-related vaccine genotype, HPV16. However, both the enumeration of memory B cells and VLP binding responses against HPV45 were poorly related to its neutralizing antibody responses. Purified IgG derived from memory B cells demonstrated specificities similar to those found in the serum, including the capacity to neutralize HPV pseudoviruses.
Conclusions: These data suggest that pseudovirus neutralization should be used as the preferred humoral immune measure for studying HPV vaccine responses, particularly for non-vaccine genotypes.
Conflict of interest statement
Figures
Similar articles
-
Neutralizing and cross-neutralizing antibody titres induced by bivalent and quadrivalent human papillomavirus vaccines in the target population of organized vaccination programmes.Vaccine. 2014 Sep 15;32(41):5357-62. doi: 10.1016/j.vaccine.2014.07.014. Epub 2014 Jul 18. Vaccine. 2014. PMID: 25045814
-
Durability of the neutralizing antibody response to vaccine and non-vaccine HPV types 7 years following immunization with either Cervarix® or Gardasil® vaccine.Vaccine. 2019 Apr 24;37(18):2455-2462. doi: 10.1016/j.vaccine.2019.03.052. Epub 2019 Mar 27. Vaccine. 2019. PMID: 30926298 Clinical Trial.
-
A randomized, observer-blinded immunogenicity trial of Cervarix(®) and Gardasil(®) Human Papillomavirus vaccines in 12-15 year old girls.PLoS One. 2013 May 1;8(5):e61825. doi: 10.1371/journal.pone.0061825. Print 2013. PLoS One. 2013. PMID: 23650505 Free PMC article. Clinical Trial.
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
-
[Research progress on the differences between T and B cell responses to three different forms of human papilloma virus (HPV) vaccination].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024 Apr;40(4):378-382. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024. PMID: 38710522 Review. Chinese.
Cited by
-
Differences in HPV-specific antibody Fc-effector functions following Gardasil® and Cervarix® vaccination.NPJ Vaccines. 2023 Mar 15;8(1):39. doi: 10.1038/s41541-023-00628-8. NPJ Vaccines. 2023. PMID: 36922512 Free PMC article.
-
AS04 drives superior cross-protective antibody response by increased NOTCH signaling of dendritic cells and proliferation of memory B cells.Front Immunol. 2025 Jul 24;16:1623405. doi: 10.3389/fimmu.2025.1623405. eCollection 2025. Front Immunol. 2025. PMID: 40808939 Free PMC article.
-
Dry Formulation of Virus-Like Particles in Electrospun Nanofibers.Vaccines (Basel). 2021 Mar 3;9(3):213. doi: 10.3390/vaccines9030213. Vaccines (Basel). 2021. PMID: 33802376 Free PMC article.
-
Selective Persistence of HPV Cross-Neutralising Antibodies following Reduced-Dose HPV Vaccine Schedules.Vaccines (Basel). 2019 Nov 28;7(4):200. doi: 10.3390/vaccines7040200. Vaccines (Basel). 2019. PMID: 31795211 Free PMC article.
-
Roles of human papillomavirus in cancers: oncogenic mechanisms and clinical use.Signal Transduct Target Ther. 2025 Jan 24;10(1):44. doi: 10.1038/s41392-024-02083-w. Signal Transduct Target Ther. 2025. PMID: 39856040 Free PMC article. Review.
References
-
- Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2010;128(4):927–35. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

