The fetal brain sparing response to hypoxia: physiological mechanisms
- PMID: 26496004
- PMCID: PMC4721497
- DOI: 10.1113/JP271099
The fetal brain sparing response to hypoxia: physiological mechanisms
Abstract
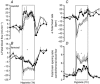
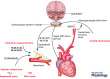
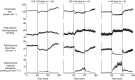
How the fetus withstands an environment of reduced oxygenation during life in the womb has been a vibrant area of research since this field was introduced by Joseph Barcroft, a century ago. Studies spanning five decades have since used the chronically instrumented fetal sheep preparation to investigate the fetal compensatory responses to hypoxia. This defence is contingent on the fetal cardiovascular system, which in late gestation adopts strategies to decrease oxygen consumption and redistribute the cardiac output away from peripheral vascular beds and towards essential circulations, such as those perfusing the brain. The introduction of simultaneous measurement of blood flow in the fetal carotid and femoral circulations by ultrasonic transducers has permitted investigation of the dynamics of the fetal brain sparing response for the first time. Now we know that major components of fetal brain sparing during acute hypoxia are triggered exclusively by a carotid chemoreflex and that they are modified by endocrine agents and the recently discovered vascular oxidant tone. The latter is determined by the interaction between nitric oxide and reactive oxygen species. The fetal brain sparing response matures as the fetus approaches term, in association with the prepartum increase in fetal plasma cortisol, and treatment of the preterm fetus with clinically relevant doses of synthetic steroids mimics this maturation. Despite intense interest into how the fetal brain sparing response may be affected by adverse intrauterine conditions, this area of research has been comparatively scant, but it is likely to take centre stage in the near future.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures

References
-
- Abrams ME, Meredith KS, Kinnard P & Clark RH (2007). Hydrops fetalis: a retrospective review of cases reported to a large national database and identification of risk factors associated with death. Pediatrics 120, 84–89. - PubMed
-
- Adams MB, Simonetta G & McMillen IC (1996). The non‐neurogenic catecholamine response of the fetal adrenal to hypoxia is dependent on activation of voltage sensitive Ca2+ channels. Brain Res Dev Brain Res 94, 182–189. - PubMed
-
- Alayed N, Kezouh A, Oddy L & Abenhaim HA (2014). Sickle cell disease and pregnancy outcomes: population‐based study on 8.8 million births. J Perinat Med 42, 487–492. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

