Multifactorial resistance to aminopeptidase inhibitor prodrug CHR2863 in myeloid leukemia cells: down-regulation of carboxylesterase 1, drug sequestration in lipid droplets and pro-survival activation ERK/Akt/mTOR
- PMID: 26496029
- PMCID: PMC4868683
- DOI: 10.18632/oncotarget.6169
Multifactorial resistance to aminopeptidase inhibitor prodrug CHR2863 in myeloid leukemia cells: down-regulation of carboxylesterase 1, drug sequestration in lipid droplets and pro-survival activation ERK/Akt/mTOR
Abstract
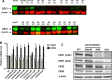
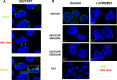
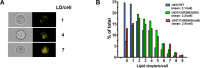
Aminopeptidase inhibitors are receiving attention as combination chemotherapeutic agents for the treatment of refractory acute myeloid leukemia. However, the factors determining therapeutic efficacy remain elusive. Here we identified the molecular basis of acquired resistance to CHR2863, an orally available hydrophobic aminopeptidase inhibitor prodrug with an esterase-sensitive motif, in myeloid leukemia cells. CHR2863 enters cells by diffusion and is retained therein upon esterase activity-mediated conversion to its hydrophilic active metabolite drug CHR6768, thereby exerting amino acid depletion. Carboxylesterases (CES) serve as candidate prodrug activating enzymes given CES1 expression in acute myeloid leukemia specimens. We established two novel myeloid leukemia sublines U937/CHR2863(200) and U937/CHR2863(5uM), with low (14-fold) and high level (270-fold) CHR2863 resistance. The latter drug resistant cells displayed: (i) complete loss of CES1-mediated drug activation associated with down-regulation of CES1 mRNA and protein, (ii) marked retention/sequestration of the prodrug, (iii) a substantial increase in intracellular lipid droplets, and (iv) a dominant activation of the pro-survival Akt/mTOR pathway. Remarkably, the latter feature coincided with a gain of sensitivity to the mTOR inhibitor rapamycin. These finding delineate the molecular basis of CHR2863 resistance and offer a novel modality to overcome this drug resistance in myeloid leukemia cells.
Keywords: aminopeptidase; carboxylesterase; lipid droplets; mTOR; rapamycin.
Conflict of interest statement
The authors declare that they have no conflict of interest pertaining to this manuscript.
Figures


Similar articles
-
Statins markedly potentiate aminopeptidase inhibitor activity against (drug-resistant) human acute myeloid leukemia cells.Cancer Drug Resist. 2023 Jul 4;6(3):430-446. doi: 10.20517/cdr.2023.20. eCollection 2023. Cancer Drug Resist. 2023. PMID: 37842233 Free PMC article.
-
Increased apoptotic efficacy of lonidamine plus arsenic trioxide combination in human leukemia cells. Reactive oxygen species generation and defensive protein kinase (MEK/ERK, Akt/mTOR) modulation.Biochem Pharmacol. 2011 Dec 1;82(11):1619-29. doi: 10.1016/j.bcp.2011.08.017. Epub 2011 Aug 27. Biochem Pharmacol. 2011. PMID: 21889928
-
Compensatory PI3-kinase/Akt/mTor activation regulates imatinib resistance development.Leukemia. 2005 Oct;19(10):1774-82. doi: 10.1038/sj.leu.2403898. Leukemia. 2005. PMID: 16136169
-
Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway.Leukemia. 2011 Jul;25(7):1064-79. doi: 10.1038/leu.2011.46. Epub 2011 Mar 25. Leukemia. 2011. PMID: 21436840 Review.
-
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.Cell Signal. 2014 Jan;26(1):149-61. doi: 10.1016/j.cellsig.2013.09.021. Epub 2013 Oct 16. Cell Signal. 2014. PMID: 24140475 Review.
Cited by
-
Aminopeptidase N: a multifunctional and promising target in medicinal chemistry.RSC Adv. 2025 Jul 23;15(32):26455-26472. doi: 10.1039/d5ra03038b. eCollection 2025 Jul 21. RSC Adv. 2025. PMID: 40703077 Free PMC article. Review.
-
The BASHY Platform Enables the Assembly of a Fluorescent Bortezomib-GV1001 Conjugate.ACS Med Chem Lett. 2021 Dec 30;13(1):128-133. doi: 10.1021/acsmedchemlett.1c00615. eCollection 2022 Jan 13. ACS Med Chem Lett. 2021. PMID: 35059132 Free PMC article.
-
Lipid droplet functions beyond energy storage.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Oct;1862(10 Pt B):1260-1272. doi: 10.1016/j.bbalip.2017.07.006. Epub 2017 Jul 19. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28735096 Free PMC article. Review.
-
Statins markedly potentiate aminopeptidase inhibitor activity against (drug-resistant) human acute myeloid leukemia cells.Cancer Drug Resist. 2023 Jul 4;6(3):430-446. doi: 10.20517/cdr.2023.20. eCollection 2023. Cancer Drug Resist. 2023. PMID: 37842233 Free PMC article.
-
The CYTOLD and ERTOLD pathways for lipid droplet-protein targeting.Trends Biochem Sci. 2022 Jan;47(1):39-51. doi: 10.1016/j.tibs.2021.08.007. Epub 2021 Sep 25. Trends Biochem Sci. 2022. PMID: 34583871 Free PMC article. Review.
References
-
- Taylor A. Aminopeptidases: structure andfunction. FASEB J. 1993;7:290–8. - PubMed
-
- Saric T, Graef CI, Goldberg AL. Pathway for degradation of peptides generated by proteasomes: a key role for thimet oligopeptidase and other metallopeptidases. J Biol Chem. 2004;279:46723–32. - PubMed
-
- Santos AN, Langner J, Herrmann M, Riemann D. Aminopeptidase N/CD13 is directly linked to signal transduction pathways in monocytes. Cell Immunol. 2000;201:22–32. - PubMed
-
- Sato Y. Role of aminopeptidase in angiogenesis. Biol Pharm Bull. 2004;27:772–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

