Double-Blind Randomized Trial of Pirfenidone in Chinese Idiopathic Pulmonary Fibrosis Patients
- PMID: 26496265
- PMCID: PMC4620844
- DOI: 10.1097/MD.0000000000001600
Double-Blind Randomized Trial of Pirfenidone in Chinese Idiopathic Pulmonary Fibrosis Patients
Abstract
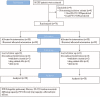
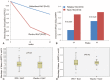
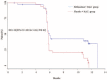
Idiopathic pulmonary fibrosis (IPF) lacks effective treatment. Pirfenidone has been used to treat IPF patients. N-acetylcysteine (NAC) exerts antioxidant and antifibrotic effects on IPF cases.This study is a double-blind, modified placebo-controlled, randomized phase II trial of pirfenidone in Chinese IPF patients. We randomly assigned the enrolled Chinese IPF patients with mild to moderate impairment of pulmonary function to receive either oral pirfenidone (1800 mg per day) and NAC (1800 mg per day) or placebo and NAC (1800 mg per day) for 48 weeks. The primary endpoints were the changes in forced vital capacity (FVC) and walking distance and the lowest SPO2 during the 6-minute walk test (6MWT) at week 48. The key secondary endpoint was the progression-free survival time. This study is registered in ClinicalTrials.gov as number NCT01504334.Eighty-six patients were screened, and 76 cases were enrolled (pirfenidone + NAC: 38; placebo + NAC: 38). The effect of pirfenidone treatment was significant at the 24th week, but this effect did not persist to the 48th week. At the 24th week, the mean decline in both FVC and ΔSPO2 (%) during the 6MWT in the pirfenidone group was lower than that in the control group (-0.08 ± 0.20 L vs -0.22 ± 0.29 L, P = 0.02 and -3.44% ± 4.51% vs -6.29% ± 6.06%, P = 0.03, respectively). However, there was no significant difference between these 2 groups at the 48th week (-0.15 ± 0.25 L vs -0.25 ± 0.28 L, P = 0.11 and -4.25% ± 7.27% vs -5.31% ± 5.49%, P = 0.51, respectively). The pirfenidone treatment group did not achieve the maximal distance difference on the 6MWT at either the 24th or the 48th week. But pirfenidone treatment prolonged the progression-free survival time in the IPF patients (hazard ratio = 1.88, 95% confidence interval: 1.092-3.242, P = 0.02). In the pirfenidone group, the adverse event (AE) rate (52.63%) was higher than that in the control group (26.3%, P = 0.03). Rash was more common in the pirfenidone group (39.5% vs 13.2%, P = 0.02).Compared with placebo combined with high-dose NAC, pirfenidone combined with high-dose NAC prolonged the progression-free survival of Chinese IPF patients with mild to moderate impairment of pulmonary function. (ClinicalTrials.gov number, NCT01504334).
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Safety and tolerability of acetylcysteine and pirfenidone combination therapy in idiopathic pulmonary fibrosis: a randomised, double-blind, placebo-controlled, phase 2 trial.Lancet Respir Med. 2016 Jun;4(6):445-53. doi: 10.1016/S2213-2600(16)30044-3. Epub 2016 May 5. Lancet Respir Med. 2016. PMID: 27161257 Clinical Trial.
-
Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials.Lancet. 2011 May 21;377(9779):1760-9. doi: 10.1016/S0140-6736(11)60405-4. Epub 2011 May 13. Lancet. 2011. PMID: 21571362 Clinical Trial.
-
A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis.N Engl J Med. 2014 May 29;370(22):2083-92. doi: 10.1056/NEJMoa1402582. Epub 2014 May 18. N Engl J Med. 2014. PMID: 24836312 Clinical Trial.
-
Pirfenidone: a review of its use in idiopathic pulmonary fibrosis.Drugs. 2015 Feb;75(2):219-30. doi: 10.1007/s40265-015-0350-9. Drugs. 2015. PMID: 25604027 Review.
-
Changing the idiopathic pulmonary fibrosis treatment approach and improving patient outcomes.Eur Respir Rev. 2012 Jun 1;21(124):161-7. doi: 10.1183/09059180.00001112. Eur Respir Rev. 2012. PMID: 22654089 Free PMC article. Review.
Cited by
-
Efficacy and safety of pirfenidone in the treatment of idiopathic pulmonary fibrosis patients: a systematic review and meta-analysis of randomised controlled trials.BMJ Open. 2021 Dec 31;11(12):e050004. doi: 10.1136/bmjopen-2021-050004. BMJ Open. 2021. PMID: 34972762 Free PMC article.
-
P311, Friend, or Foe of Tissue Fibrosis?Front Pharmacol. 2018 Oct 12;9:1151. doi: 10.3389/fphar.2018.01151. eCollection 2018. Front Pharmacol. 2018. PMID: 30369881 Free PMC article. Review.
-
Comparison of Longevity in Patients with Idiopathic Pulmonary Fibrosis Using Pirfenidone Versus Triple Therapy with Prednisolone, Azathioprine, and Acetylcysteine.Tanaffos. 2023 Jan;22(1):129-135. Tanaffos. 2023. PMID: 37920315 Free PMC article.
-
Efficacy of Combination Therapy With Pirfenidone and Low-Dose Cyclophosphamide for Refractory Interstitial Lung Disease Associated With Connective Tissue Disease: A Case-Series of Seven Patients.Arch Rheumatol. 2019 Aug 26;35(2):180-188. doi: 10.46497/ArchRheumatol.2020.7381. eCollection 2020 Jun. Arch Rheumatol. 2019. PMID: 32851366 Free PMC article.
-
A CARE-compliant case report: Lung transplantation for a Chinese young man with idiopathic pleuroparenchymal fibroelastosis.Medicine (Baltimore). 2017 May;96(19):e6900. doi: 10.1097/MD.0000000000006900. Medicine (Baltimore). 2017. PMID: 28489801 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

