Sequential release of nanoparticle payloads from ultrasonically burstable capsules
- PMID: 26496382
- PMCID: PMC4685712
- DOI: 10.1016/j.biomaterials.2015.10.008
Sequential release of nanoparticle payloads from ultrasonically burstable capsules
Abstract
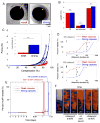
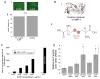
In many biomedical contexts ranging from chemotherapy to tissue engineering, it is beneficial to sequentially present bioactive payloads. Explicit control over the timing and dose of these presentations is highly desirable. Here, we present a capsule-based delivery system capable of rapidly releasing multiple payloads in response to ultrasonic signals. In vitro, these alginate capsules exhibited excellent payload retention for up to 1 week when unstimulated and delivered their entire payloads when ultrasonically stimulated for 10-100 s. Shorter exposures (10 s) were required to trigger delivery from capsules embedded in hydrogels placed in a tissue model and did not result in tissue heating or death of encapsulated cells. Different types of capsules were tuned to rupture in response to different ultrasonic stimuli, thus permitting the sequential, on-demand delivery of nanoparticle payloads. As a proof of concept, gold nanoparticles were decorated with bone morphogenetic protein-2 to demonstrate the potential bioactivity of nanoparticle payloads. These nanoparticles were not cytotoxic and induced an osteogenic response in mouse mesenchymal stem cells. This system may enable researchers and physicians to remotely regulate the timing, dose, and sequence of drug delivery on-demand, with a wide range of clinical applications ranging from tissue engineering to cancer treatment.
Keywords: Biomaterials; Controlled release; Drug delivery; Ultrasound response.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Ultrasonic Generation of Pulsatile and Sequential Therapeutic Delivery Profiles from Calcium-Crosslinked Alginate Hydrogels.Molecules. 2019 Mar 16;24(6):1048. doi: 10.3390/molecules24061048. Molecules. 2019. PMID: 30884862 Free PMC article.
-
Switchable Release of Entrapped Nanoparticles from Alginate Hydrogels.Adv Healthc Mater. 2015 Aug 5;4(11):1634-1639. doi: 10.1002/adhm.201500254. Epub 2015 Jun 5. Adv Healthc Mater. 2015. PMID: 26044285 Free PMC article.
-
Osteogenic/angiogenic dual growth factor delivery microcapsules for regeneration of vascularized bone tissue.Adv Healthc Mater. 2015 Sep 16;4(13):1982-92. doi: 10.1002/adhm.201500341. Epub 2015 Jul 2. Adv Healthc Mater. 2015. PMID: 26138344
-
Interpenetrating Polymer Networks polysaccharide hydrogels for drug delivery and tissue engineering.Adv Drug Deliv Rev. 2013 Aug;65(9):1172-87. doi: 10.1016/j.addr.2013.04.002. Epub 2013 Apr 17. Adv Drug Deliv Rev. 2013. PMID: 23603210 Review.
-
Mesenchymal stem cells and alginate microcarriers for craniofacial bone tissue engineering: A review.J Biomed Mater Res A. 2016 May;104(5):1276-84. doi: 10.1002/jbm.a.35647. Epub 2016 Jan 29. J Biomed Mater Res A. 2016. PMID: 26826060 Review.
Cited by
-
Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications.Mater Today Bio. 2021 Dec 9;13:100186. doi: 10.1016/j.mtbio.2021.100186. eCollection 2022 Jan. Mater Today Bio. 2021. PMID: 34917924 Free PMC article. Review.
-
Lyophilized Drug-Loaded Solid Lipid Nanoparticles Formulated with Beeswax and Theobroma Oil.Molecules. 2021 Feb 9;26(4):908. doi: 10.3390/molecules26040908. Molecules. 2021. PMID: 33572168 Free PMC article.
-
In vitro and in vivo assessment of controlled release and degradation of acoustically responsive scaffolds.Acta Biomater. 2016 Dec;46:221-233. doi: 10.1016/j.actbio.2016.09.026. Epub 2016 Sep 27. Acta Biomater. 2016. PMID: 27686040 Free PMC article.
-
Active biomaterials for mechanobiology.Biomaterials. 2021 Jan;267:120497. doi: 10.1016/j.biomaterials.2020.120497. Epub 2020 Oct 26. Biomaterials. 2021. PMID: 33129187 Free PMC article. Review.
-
Recent advances in light-responsive on-demand drug-delivery systems.Ther Deliv. 2017 Feb;8(2):89-107. doi: 10.4155/tde-2016-0060. Ther Deliv. 2017. PMID: 28088880 Free PMC article. Review.
References
-
- Peppas NA, Leobandung W. Stimuli-responsive hydrogels: Ideal carriers for chronobiology and chronotherapy. J Biomater Sci Polym Ed. 2004;15(2):125–44. - PubMed
-
- Mormont MC, Levi F. Cancer chronotherapy: Principles, applications, and perspectives. Cancer. 2003;97(1):155–69. - PubMed
-
- Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

