Timescales and Frequencies of Reversible and Irreversible Adhesion Events of Single Bacterial Cells
- PMID: 26496389
- PMCID: PMC4756760
- DOI: 10.1021/acs.analchem.5b02087
Timescales and Frequencies of Reversible and Irreversible Adhesion Events of Single Bacterial Cells
Abstract
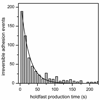
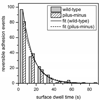
In the environment, most bacteria form surface-attached cell communities called biofilms. The attachment of single cells to surfaces involves an initial reversible stage typically mediated by surface structures such as flagella and pili, followed by a permanent adhesion stage usually mediated by polysaccharide adhesives. Here, we determine the absolute and relative timescales and frequencies of reversible and irreversible adhesion of single cells of the bacterium Caulobacter crescentus to a glass surface in a microfluidic device. We used fluorescence microscopy of C. crescentus expressing green fluorescent protein to track the swimming behavior of individual cells prior to adhesion, monitor the cell at the surface, and determine whether the cell reversibly or irreversibly adhered to the surface. A fluorescently labeled lectin that binds specifically to polar polysaccharides, termed holdfast, discriminated irreversible adhesion events from reversible adhesion events where no holdfast formed. In wild-type cells, the holdfast production time for irreversible adhesion events initiated by surface contact (23 s) was 30-times faster than the holdfast production time that occurs through developmental regulation (13 min). Irreversible adhesion events in wild-type cells (3.3 events/min) are 15-times more frequent than in pilus-minus mutant cells (0.2 events/min), indicating the pili are critical structures in the transition from reversible to irreversible surface-stimulated adhesion. In reversible adhesion events, the dwell time of cells at the surface before departing was the same for wild-type cells (12 s) and pilus-minus mutant cells (13 s), suggesting the pili do not play a significant role in reversible adhesion. Moreover, reversible adhesion events in wild-type cells (6.8 events/min) occur twice as frequently as irreversible adhesion events (3.3 events/min), demonstrating that most cells contact the surface multiple times before transitioning from reversible to irreversible adhesion.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

