Monitoring of the Parasite Load in the Digestive Tract of Rhodnius prolixus by Combined qPCR Analysis and Imaging Techniques Provides New Insights into the Trypanosome Life Cycle
- PMID: 26496442
- PMCID: PMC4619730
- DOI: 10.1371/journal.pntd.0004186
Monitoring of the Parasite Load in the Digestive Tract of Rhodnius prolixus by Combined qPCR Analysis and Imaging Techniques Provides New Insights into the Trypanosome Life Cycle
Abstract
Background: Here we report the monitoring of the digestive tract colonization of Rhodnius prolixus by Trypanosoma cruzi using an accurate determination of the parasite load by qPCR coupled with fluorescence and bioluminescence imaging (BLI). These complementary methods revealed critical steps necessary for the parasite population to colonize the insect gut and establish vector infection.
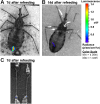
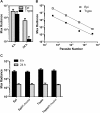
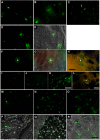
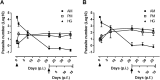
Methodology/principal findings: qPCR analysis of the parasite load in the insect gut showed several limitations due mainly to the presence of digestive-derived products that are thought to degrade DNA and inhibit further the PCR reaction. We developed a real-time PCR strategy targeting the T. cruzi repetitive satellite DNA sequence using as internal standard for normalization, an exogenous heterologous DNA spiked into insect samples extract, to precisely quantify the parasite load in each segment of the insect gut (anterior midgut, AM, posterior midgut, PM, and hindgut, H). Using combined fluorescence microscopy and BLI imaging as well as qPCR analysis, we showed that during their journey through the insect digestive tract, most of the parasites are lysed in the AM during the first 24 hours independently of the gut microbiota. During this short period, live parasites move through the PM to establish the onset of infection. At days 3-4 post-infection (p.i.), the parasite population begins to colonize the H to reach a climax at day 7 p.i., which is maintained during the next two weeks. Remarkably, the fluctuation of the parasite number in H remains relatively stable over the two weeks after refeeding, while the populations residing in the AM and PM increases slightly and probably constitutes the reservoirs of dividing epimastigotes.
Conclusions/significance: These data show that a tuned dynamic control of the population operates in the insect gut to maintain an equilibrium between non-dividing infective trypomastigote forms and dividing epimastigote forms of the parasite, which is crucial for vector competence.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Maguire JH (2006) Chagas' disease—can we stop the deaths? N Engl J Med 355: 760–761. - PubMed
-
- Teixeira AR, Nascimento RJ, Sturm NR (2006) Evolution and pathology in chagas disease—a review. Mem Inst Oswaldo Cruz 101: 463–491. - PubMed
-
- Kollien AH, Schaub GA (2000) The development of Trypanosoma cruzi in triatominae. Parasitol Today 16: 381–387. - PubMed
-
- Wigglesworth VB (1972) The principies of insect physiology 7th ed. London: Chapman & Hall. 827 p.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

