GLT1 overexpression reverses established neuropathic pain-related behavior and attenuates chronic dorsal horn neuron activation following cervical spinal cord injury
- PMID: 26496514
- PMCID: PMC4703512
- DOI: 10.1002/glia.22936
GLT1 overexpression reverses established neuropathic pain-related behavior and attenuates chronic dorsal horn neuron activation following cervical spinal cord injury
Abstract
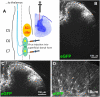
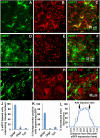
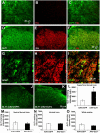
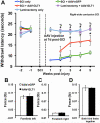
Development of neuropathic pain occurs in a major portion of traumatic spinal cord injury (SCI) patients, resulting in debilitating and often long-term physical and psychological burdens. Following SCI, chronic dysregulation of extracellular glutamate homeostasis has been shown to play a key role in persistent central hyperexcitability of superficial dorsal horn neurons that mediate pain neurotransmission, leading to various forms of neuropathic pain. Astrocytes express the major CNS glutamate transporter, GLT1, which is responsible for the vast majority of functional glutamate uptake, particularly in the spinal cord. In our unilateral cervical contusion model of mouse SCI that is associated with ipsilateral forepaw heat hypersensitivity (a form of chronic at-level neuropathic pain-related behavior), we previously reported significant and long-lasting reductions in GLT1 expression and functional GLT1-mediated glutamate uptake in cervical spinal cord dorsal horn. To therapeutically address GLT1 dysfunction following cervical contusion SCI, we injected an adeno-associated virus type 8 (AAV8)-Gfa2 vector into the superficial dorsal horn to increase GLT1 expression selectively in astrocytes. Compared to both contusion-only animals and injured mice that received AAV8-eGFP control injection, AAV8-GLT1 delivery increased GLT1 protein expression in astrocytes of the injured cervical spinal cord dorsal horn, resulting in a significant and persistent reversal of already-established heat hypersensitivity. Furthermore, AAV8-GLT1 injection significantly reduced expression of the transcription factor and marker of persistently increased neuronal activation, ΔFosB, in superficial dorsal horn neurons. These results demonstrate that focal restoration of GLT1 expression in the superficial dorsal horn is a promising target for treating chronic neuropathic pain following SCI.
Keywords: adeno-associated virus; astrocyte; contusion; glutamate transporter; neuropathic pain.
© 2015 Wiley Periodicals, Inc.
Figures
Similar articles
-
Chronic at-level thermal hyperalgesia following rat cervical contusion spinal cord injury is accompanied by neuronal and astrocyte activation and loss of the astrocyte glutamate transporter, GLT1, in superficial dorsal horn.Brain Res. 2014 Sep 18;1581:64-79. doi: 10.1016/j.brainres.2014.05.003. Epub 2014 May 14. Brain Res. 2014. PMID: 24833066 Free PMC article.
-
Persistent at-level thermal hyperalgesia and tactile allodynia accompany chronic neuronal and astrocyte activation in superficial dorsal horn following mouse cervical contusion spinal cord injury.PLoS One. 2014 Sep 30;9(9):e109099. doi: 10.1371/journal.pone.0109099. eCollection 2014. PLoS One. 2014. PMID: 25268642 Free PMC article.
-
Overexpression of the astrocyte glutamate transporter GLT1 exacerbates phrenic motor neuron degeneration, diaphragm compromise, and forelimb motor dysfunction following cervical contusion spinal cord injury.J Neurosci. 2014 May 28;34(22):7622-38. doi: 10.1523/JNEUROSCI.4690-13.2014. J Neurosci. 2014. PMID: 24872566 Free PMC article.
-
Neuronal hyperexcitability: a substrate for central neuropathic pain after spinal cord injury.Curr Pain Headache Rep. 2011 Jun;15(3):215-22. doi: 10.1007/s11916-011-0186-2. Curr Pain Headache Rep. 2011. PMID: 21387163 Review.
-
Neuronal-Glial Interactions Maintain Chronic Neuropathic Pain after Spinal Cord Injury.Neural Plast. 2017;2017:2480689. doi: 10.1155/2017/2480689. Epub 2017 Aug 29. Neural Plast. 2017. PMID: 28951789 Free PMC article. Review.
Cited by
-
The Role of Astrocytes in the Modulation ofK+-Cl--Cotransporter-2 Function.Int J Mol Sci. 2020 Dec 15;21(24):9539. doi: 10.3390/ijms21249539. Int J Mol Sci. 2020. PMID: 33333849 Free PMC article. Review.
-
EphrinB2 knockdown in cervical spinal cord preserves diaphragm innervation in a mutant SOD1 mouse model of ALS.Elife. 2024 Jan 15;12:RP89298. doi: 10.7554/eLife.89298. Elife. 2024. PMID: 38224498 Free PMC article.
-
Ventrolateral Periaqueductal Gray Astrocytes Regulate Nociceptive Sensation and Emotional Motivation in Diabetic Neuropathic Pain.J Neurosci. 2022 Oct 26;42(43):8184-8199. doi: 10.1523/JNEUROSCI.0920-22.2022. Epub 2022 Sep 15. J Neurosci. 2022. PMID: 36109166 Free PMC article.
-
Biomimetic Scaffolds Enhance iPSC Astrocyte Progenitor Angiogenic, Immunomodulatory, and Neurotrophic Capacity in a Stiffness and Matrix-Dependent Manner for Spinal Cord Repair Applications.Adv Healthc Mater. 2025 Jun;14(16):e2500830. doi: 10.1002/adhm.202500830. Epub 2025 May 19. Adv Healthc Mater. 2025. PMID: 40384159 Free PMC article.
-
How is chronic pain related to sympathetic dysfunction and autonomic dysreflexia following spinal cord injury?Auton Neurosci. 2018 Jan;209:79-89. doi: 10.1016/j.autneu.2017.01.006. Epub 2017 Jan 27. Auton Neurosci. 2018. PMID: 28161248 Free PMC article. Review.
References
-
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. - PubMed
-
- Baastrup C, Finnerup NB. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22:455–75. - PubMed
-
- Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. - PubMed
-
- Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev. 2008;32:972–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

