The Direct Actions of GABA, 2'-Methoxy-6-Methylflavone and General Anaesthetics at β3γ2L GABAA Receptors: Evidence for Receptors with Different Subunit Stoichiometries
- PMID: 26496640
- PMCID: PMC4619705
- DOI: 10.1371/journal.pone.0141359
The Direct Actions of GABA, 2'-Methoxy-6-Methylflavone and General Anaesthetics at β3γ2L GABAA Receptors: Evidence for Receptors with Different Subunit Stoichiometries
Abstract
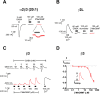
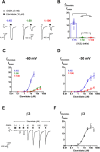
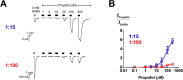
2'-Methoxy-6-methylflavone (2'MeO6MF) is an anxiolytic flavonoid which has been shown to display GABAA receptor (GABAAR) β2/3-subunit selectivity, a pharmacological profile similar to that of the general anaesthetic etomidate. Electrophysiological studies suggest that the full agonist action of 2'MeO6MF at α2β3γ2L GABAARs may mediate the flavonoid's in vivo effects. However, we found variations in the relative efficacy of 2'MeO6MF (2'MeO6MF-elicited current responses normalised to the maximal GABA response) at α2β3γ2L GABAARs due to the presence of mixed receptor populations. To understand which receptor subpopulation(s) underlie the variations observed, we conducted a systematic investigation of 2'MeO6MF activity at all receptor combinations that could theoretically form (α2, β3, γ2L, α2β3, α2γ2L, β3γ2L and α2β3γ2L) in Xenopus oocytes using the two-electrode voltage clamp technique. We found that 2'MeO6MF activated non-α-containing β3γ2L receptors. In an attempt to establish the optimal conditions to express a uniform population of these receptors, we found that varying the relative amounts of β3:γ2L subunit mRNAs resulted in differences in the level of constitutive activity, the GABA concentration-response relationships, and the relative efficacy of 2'MeO6MF activation. Like 2'MeO6MF, general anaesthetics such as etomidate and propofol also showed distinct levels of relative efficacy across different injection ratios. Based on these results, we infer that β3γ2L receptors may form with different subunit stoichiometries, resulting in the complex pharmacology observed across different injection ratios. Moreover, the discovery that GABA and etomidate have direct actions at the α-lacking β3γ2L receptors raises questions about the structural requirements for their respective binding sites at GABAARs.
Conflict of interest statement
Figures



Similar articles
-
3-Hydroxy-2'-methoxy-6-methylflavone: a potent anxiolytic with a unique selectivity profile at GABA(A) receptor subtypes.Biochem Pharmacol. 2011 Dec 15;82(12):1971-83. doi: 10.1016/j.bcp.2011.09.002. Epub 2011 Sep 8. Biochem Pharmacol. 2011. PMID: 21924247
-
2'-Methoxy-6-methylflavone: a novel anxiolytic and sedative with subtype selective activating and modulating actions at GABA(A) receptors.Br J Pharmacol. 2012 Feb;165(4):880-96. doi: 10.1111/j.1476-5381.2011.01604.x. Br J Pharmacol. 2012. PMID: 21797842 Free PMC article.
-
The interaction of general anaesthetics with recombinant GABAA and glycine receptors expressed in Xenopus laevis oocytes: a comparative study.Br J Pharmacol. 1997 Dec;122(8):1707-19. doi: 10.1038/sj.bjp.0701563. Br J Pharmacol. 1997. PMID: 9422818 Free PMC article.
-
The Role of GABA Receptors in Anesthesia and Sedation: An Updated Review.CNS Drugs. 2025 Jan;39(1):39-54. doi: 10.1007/s40263-024-01128-6. Epub 2024 Oct 27. CNS Drugs. 2025. PMID: 39465449 Free PMC article. Review.
-
Closing the gap between the molecular and systemic actions of anesthetic agents.Adv Pharmacol. 2015;72:229-62. doi: 10.1016/bs.apha.2014.10.009. Epub 2014 Dec 4. Adv Pharmacol. 2015. PMID: 25600373 Review.
Cited by
-
α subunits in GABAA receptors are dispensable for GABA and diazepam action.Sci Rep. 2017 Nov 14;7(1):15498. doi: 10.1038/s41598-017-15628-7. Sci Rep. 2017. PMID: 29138471 Free PMC article.
-
Zolpidem is a potent stoichiometry-selective modulator of α1β3 GABAA receptors: evidence of a novel benzodiazepine site in the α1-α1 interface.Sci Rep. 2016 Jun 27;6:28674. doi: 10.1038/srep28674. Sci Rep. 2016. PMID: 27346730 Free PMC article.
-
Concatenated γ-aminobutyric acid type A receptors revisited: Finding order in chaos.J Gen Physiol. 2019 Jun 3;151(6):798-819. doi: 10.1085/jgp.201812133. Epub 2019 Apr 15. J Gen Physiol. 2019. PMID: 30988061 Free PMC article.
-
Genistein and Vanadate Differentially Modulate Cortical GABAA Receptor/ATPase Activity and Behavior in Rats via a Phenol-Sensitive Mechanism.Int J Mol Sci. 2025 Jun 15;26(12):5731. doi: 10.3390/ijms26125731. Int J Mol Sci. 2025. PMID: 40565196 Free PMC article.
-
Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions.Epilepsia. 2019 Nov;60(11):2224-2234. doi: 10.1111/epi.16355. Epub 2019 Oct 17. Epilepsia. 2019. PMID: 31625159 Free PMC article.
References
-
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nature Reviews Neuroscience. 2005;6(3):215–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

