Application of retinoic acid improves form and function of tissue engineered corneal construct
- PMID: 26496651
- PMCID: PMC4879898
- DOI: 10.1080/15476278.2015.1093267
Application of retinoic acid improves form and function of tissue engineered corneal construct
Abstract
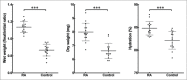
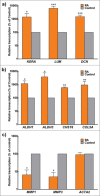
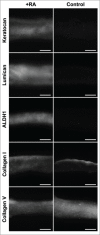
Retinoic acid has recently been shown to control the phenotype and extracellular matrix composition of corneal stromal cells cultured in vitro as monolayers. This study set out to investigate the effects of retinoic acid on human corneal keratocytes within a 3D environment. Human corneal keratocytes were encapsulated in collagen gels, which were subsequently compressed under load, and cultured in serum-free media supplemented with 10 µM retinoic acid or DMSO vehicle for 30 days. Cell proliferation was quantified on selected days, while the expression of several important keratocytes markers was evaluated at day 30 using RT-PCR and immunoblotting. The weight and size of the collagen constructs were measured before and after hydration and contraction analyses. Retinoic acid enhanced keratocyte proliferation until day 30, whereas cells in control culture conditions showed reduced numbers after day 21. Both gene and protein expressions of keratocyte-characteristic proteoglycans (keratocan, lumican and decorin), corneal crystallins and collagen type I and V were significantly increased following retinoic acid supplementation. Retinoic acid also significantly reduced the expression of matrix metalloproteases 1, 3 and 9 while not increasing α-smooth muscle actin and fibronectin expression. Furthermore, these effects were also correlated with the ability of retinoic acid to significantly inhibit the contractility of keratocytes while allowing the build-up of corneal stromal extracellular matrix within the 3D constructs. Thus, retinoic acid supplementation represents a promising strategy to improve the phenotype of 3D-cultured keratocytes, and their usefulness as a model of corneal stroma for corneal biology and regenerative medicine applications.
Keywords: 3D model; Cornea; collagen gel; in vitro; keratocytes; retinoic acid; tissue engineering.
Figures



Similar articles
-
The effects of retinoic acid on human corneal stromal keratocytes cultured in vitro under serum-free conditions.Invest Ophthalmol Vis Sci. 2013 Nov 13;54(12):7483-91. doi: 10.1167/iovs.13-13092. Invest Ophthalmol Vis Sci. 2013. PMID: 24150763
-
The effect of growth factor supplementation on corneal stromal cell phenotype in vitro using a serum-free media.Exp Eye Res. 2016 Oct;151:26-37. doi: 10.1016/j.exer.2016.07.015. Epub 2016 Jul 22. Exp Eye Res. 2016. PMID: 27456135
-
Corneal keratocyte transition to mesenchymal stem cell phenotype and reversal using serum-free medium supplemented with fibroblast growth factor-2, transforming growth factor-β3 and retinoic acid.J Tissue Eng Regen Med. 2018 Jan;12(1):e203-e215. doi: 10.1002/term.2316. Epub 2017 Mar 30. J Tissue Eng Regen Med. 2018. PMID: 27685949
-
Keratocyte biology.Exp Eye Res. 2020 Jul;196:108062. doi: 10.1016/j.exer.2020.108062. Epub 2020 May 19. Exp Eye Res. 2020. PMID: 32442558 Review.
-
Keratocyte mechanobiology.Exp Eye Res. 2020 Nov;200:108228. doi: 10.1016/j.exer.2020.108228. Epub 2020 Sep 10. Exp Eye Res. 2020. PMID: 32919993 Free PMC article. Review.
Cited by
-
Retinoic Acid Enhances the Differentiation of Adipose-Derived Stem Cells to Keratocytes In Vitro.Transl Vis Sci Technol. 2017 Jan 20;6(1):6. doi: 10.1167/tvst.6.1.6. eCollection 2017 Jan. Transl Vis Sci Technol. 2017. PMID: 28138416 Free PMC article.
-
3D Printing of Tissue Engineered Constructs for In Vitro Modeling of Disease Progression and Drug Screening.Ann Biomed Eng. 2017 Jan;45(1):164-179. doi: 10.1007/s10439-016-1640-4. Epub 2016 May 11. Ann Biomed Eng. 2017. PMID: 27169894 Free PMC article. Review.
-
Application of drug delivery systems for the controlled delivery of growth factors to treat nervous system injury.Organogenesis. 2018;14(3):123-128. doi: 10.1080/15476278.2018.1491183. Epub 2018 Aug 27. Organogenesis. 2018. PMID: 30148412 Free PMC article. Review.
-
Retinoic acid promotes in vitro follicle activation in the cat ovary by regulating expression of matrix metalloproteinase 9.PLoS One. 2018 Aug 24;13(8):e0202759. doi: 10.1371/journal.pone.0202759. eCollection 2018. PLoS One. 2018. PMID: 30142172 Free PMC article.
-
Serum Vitamin A Levels May Affect the Severity of Ocular Graft-versus-Host Disease.Front Med (Lausanne). 2017 Jun 22;4:67. doi: 10.3389/fmed.2017.00067. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28691006 Free PMC article.
References
-
- Griffith M, Hakim M, Shimmura S, Watsky MA, Li F, Carlsson D, Doillon CJ, Nakamura M, Suuronen E, Shinozaki N, et al.. Artificial human corneas: scaffolds for transplantation and host regeneration. Cornea 2002; 21:S54-S61; PMID:12484700; http://dx.doi.org/10.1097/01.ico.0000263120.68768.f8 - DOI - PubMed
-
- Ruberti JW, Zieske JD. Prelude to corneal tissue engineering–gaining control of collagen organization. Prog Retin Eye Res 2008; 27:549-77; PMID:18775789; http://dx.doi.org/10.1016/j.preteyeres.2008.08.001 - DOI - PMC - PubMed
-
- Ousley PJ, Terry MA. Objective screening methods for prior refractive surgery in donor tissue. Cornea 2002; 21:181-8; PMID:11862091; http://dx.doi.org/10.1097/00003226-200203000-00011 - DOI - PubMed
-
- Germain L, Carrier P, Auger FA, Salesse C, Guérin SL. Can we produce a human corneal equivalent by tissue engineering? Prog Retin Eye Res 2000; 19:497-527; PMID:10925241; http://dx.doi.org/10.1016/S1350-9462(00)00005-7 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
