Energy Landscape of Alginate-Epimerase Interactions Assessed by Optical Tweezers and Atomic Force Microscopy
- PMID: 26496653
- PMCID: PMC4619708
- DOI: 10.1371/journal.pone.0141237
Energy Landscape of Alginate-Epimerase Interactions Assessed by Optical Tweezers and Atomic Force Microscopy
Abstract
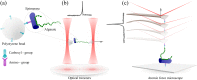
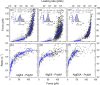
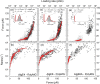
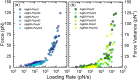
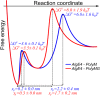
Mannuronan C-5 epimerases are a family of enzymes that catalyze epimerization of alginates at the polymer level. This group of enzymes thus enables the tailor-making of various alginate residue sequences to attain various functional properties, e.g. viscosity, gelation and ion binding. Here, the interactions between epimerases AlgE4 and AlgE6 and alginate substrates as well as epimerization products were determined. The interactions of the various epimerase-polysaccharide pairs were determined over an extended range of force loading rates by the combined use of optical tweezers and atomic force microscopy. When studying systems that in nature are not subjected to external forces the access to observations obtained at low loading rates, as provided by optical tweezers, is a great advantage since the low loading rate region for these systems reflect the properties of the rate limiting energy barrier. The AlgE epimerases have a modular structure comprising both A and R modules, and the role of each of these modules in the epimerization process were examined through studies of the A- module of AlgE6, AlgE6A. Dynamic strength spectra obtained through combination of atomic force microscopy and the optical tweezers revealed the existence of two energy barriers in the alginate-epimerase complexes, of which one was not revealed in previous AFM based studies of these complexes. Furthermore, based on these spectra estimates of the locations of energy transition states (xβ), lifetimes in the absence of external perturbation (τ0) and free energies (ΔG#) were determined for the different epimerase-alginate complexes. This is the first determination of ΔG# for these complexes. The values determined were up to 8 kBT for the outer barrier, and smaller values for the inner barriers. The size of the free energies determined are consistent with the interpretation that the enzyme and substrate are thus not tightly locked at all times but are able to relocate. Together with the observed different affinities determined for AlgE4-polymannuronic acid (poly-M) and AlgE4-polyalternating alginate (poly-MG) macromolecular pairs these data give important contribution to the growing understanding of the mechanisms underlying the processive mode of these enzymes.
Conflict of interest statement
Figures


References
-
- Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49(8):1993–2007. 10.1016/j.polymer.2008.01.027 - DOI
-
- Lim F, Sun AM. Microencapsulated islets as a bioartificial endocrine pancreas. Science. 1980;210:908–10. - PubMed
-
- Rozeboom HJ, Bjerkan TM, Kalk KH, Ertesvag H, Holtan S, Aachmann FL, et al. Structural and mutational characterization of the catalytic A-module of the mannuronan C-5-epimerase AlgE4 from Azotobacter vinelandii. Journal of Biological Chemistry. 2008;283(35):23819–28. 10.1074/jbc.M804119200 . - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

