Developmental Effects of the ToxCast™ Phase I and Phase II Chemicals in Caenorhabditis elegans and Corresponding Responses in Zebrafish, Rats, and Rabbits
- PMID: 26496690
- PMCID: PMC4858399
- DOI: 10.1289/ehp.1409645
Developmental Effects of the ToxCast™ Phase I and Phase II Chemicals in Caenorhabditis elegans and Corresponding Responses in Zebrafish, Rats, and Rabbits
Abstract
Background: Modern toxicology is shifting from an observational to a mechanistic science. As part of this shift, high-throughput toxicity assays are being developed using alternative, nonmammalian species to prioritize chemicals and develop prediction models of human toxicity.
Methods: The nematode Caenorhabditis elegans (C. elegans) was used to screen the U.S. Environmental Protection Agency's (EPA's) ToxCast™ Phase I and Phase II libraries, which contain 292 and 676 chemicals, respectively, for chemicals leading to decreased larval development and growth. Chemical toxicity was evaluated using three parameters: a biologically defined effect size threshold, half-maximal activity concentration (AC50), and lowest effective concentration (LEC).
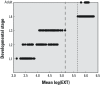
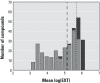
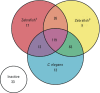
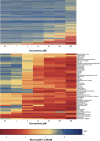
Results: Across both the Phase I and Phase II libraries, 62% of the chemicals were classified as active ≤ 200 μM in the C. elegans assay. Chemical activities and potencies in C. elegans were compared with those from two zebrafish embryonic development toxicity studies and developmental toxicity data for rats and rabbits. Concordance of chemical activity was higher between C. elegans and one zebrafish assay across Phase I chemicals (79%) than with a second zebrafish assay (59%). Using C. elegans or zebrafish to predict rat or rabbit developmental toxicity resulted in balanced accuracies (the average value of the sensitivity and specificity for an assay) ranging from 45% to 53%, slightly lower than the concordance between rat and rabbit (58%).
Conclusions: Here, we present an assay that quantitatively and reliably describes the effects of chemical toxicants on C. elegans growth and development. We found significant overlap in the activity of chemicals in the ToxCast™ libraries between C. elegans and zebrafish developmental screens. Incorporating C. elegans toxicological assays as part of a battery of in vitro and in vivo assays provides additional information for the development of models to predict a chemical's potential toxicity to humans.
Citation: Boyd WA, Smith MV, Co CA, Pirone JR, Rice JR, Shockley KR, Freedman JH. 2016. Developmental effects of the ToxCast™ Phase I and II chemicals in Caenorhabditis elegans and corresponding responses in zebrafish, rats, and rabbits. Environ Health Perspect 124:586-593; http://dx.doi.org/10.1289/ehp.1409645.
Conflict of interest statement
The authors declare they have no actual or potential competing financial interests.
Figures
Similar articles
-
A Comparison of ToxCast Test Results with In Vivo and Other In Vitro Endpoints for Neuro, Endocrine, and Developmental Toxicities: A Case Study Using Endosulfan and Methidathion.Birth Defects Res B Dev Reprod Toxicol. 2015 Apr;104(2):71-89. doi: 10.1002/bdrb.21140. Epub 2015 May 27. Birth Defects Res B Dev Reprod Toxicol. 2015. PMID: 26017137
-
Screening and characterization of 133 physiologically-relevant environmental chemicals for reproductive toxicity.Reprod Toxicol. 2024 Jun;126:108602. doi: 10.1016/j.reprotox.2024.108602. Epub 2024 May 8. Reprod Toxicol. 2024. PMID: 38723698 Free PMC article.
-
Environmental impact on vascular development predicted by high-throughput screening.Environ Health Perspect. 2011 Nov;119(11):1596-603. doi: 10.1289/ehp.1103412. Epub 2011 Jul 25. Environ Health Perspect. 2011. PMID: 21788198 Free PMC article.
-
Zebrafish: as an integrative model for twenty-first century toxicity testing.Birth Defects Res C Embryo Today. 2011 Sep;93(3):256-67. doi: 10.1002/bdrc.20214. Birth Defects Res C Embryo Today. 2011. PMID: 21932434 Review.
-
Predictive models and computational toxicology.Methods Mol Biol. 2013;947:343-74. doi: 10.1007/978-1-62703-131-8_26. Methods Mol Biol. 2013. PMID: 23138916 Review.
Cited by
-
Mitochondria as a target of organophosphate and carbamate pesticides: Revisiting common mechanisms of action with new approach methodologies.Reprod Toxicol. 2019 Oct;89:83-92. doi: 10.1016/j.reprotox.2019.07.007. Epub 2019 Jul 14. Reprod Toxicol. 2019. PMID: 31315019 Free PMC article.
-
Leveraging open cheminformatics tools for non-targeted metabolomics analysis of C. elegans: a workflow comparison and application to strains related to xenobiotic metabolism and neurodegeneration.Anal Bioanal Chem. 2025 Aug 8. doi: 10.1007/s00216-025-06048-y. Online ahead of print. Anal Bioanal Chem. 2025. PMID: 40775120
-
Prioritization of chemicals in food for risk assessment by integrating exposure estimates and new approach methodologies: A next generation risk assessment case study.Front Toxicol. 2022 Sep 19;4:933197. doi: 10.3389/ftox.2022.933197. eCollection 2022. Front Toxicol. 2022. PMID: 36199824 Free PMC article.
-
Caenorhabditis elegans as a Model to Study Manganese-Induced Neurotoxicity.Biomolecules. 2022 Sep 29;12(10):1396. doi: 10.3390/biom12101396. Biomolecules. 2022. PMID: 36291605 Free PMC article. Review.
-
Towards improved screening of toxins for Parkinson's risk.NPJ Parkinsons Dis. 2023 Dec 19;9(1):169. doi: 10.1038/s41531-023-00615-9. NPJ Parkinsons Dis. 2023. PMID: 38114496 Free PMC article. Review.
References
-
- Benson JA, Cummings EE, O’Reilly LP, Lee MH, Pak SC. A high-content assay for identifying small molecules that reprogram C. elegans germ cell fate. Methods. 2014;68:529–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

