Measurement of Systemic Mitochondrial Function in Advanced Primary Open-Angle Glaucoma and Leber Hereditary Optic Neuropathy
- PMID: 26496696
- PMCID: PMC4619697
- DOI: 10.1371/journal.pone.0140919
Measurement of Systemic Mitochondrial Function in Advanced Primary Open-Angle Glaucoma and Leber Hereditary Optic Neuropathy
Abstract
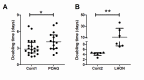
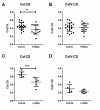
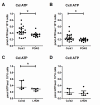
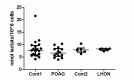
Primary Open Angle Glaucoma (POAG) is a common neurodegenerative disease characterized by the selective and gradual loss of retinal ganglion cells (RGCs). Aging and increased intraocular pressure (IOP) are glaucoma risk factors; nevertheless patients deteriorate at all levels of IOP, implying other causative factors. Recent evidence presents mitochondrial oxidative phosphorylation (OXPHOS) complex-I impairments in POAG. Leber Hereditary Optic Neuropathy (LHON) patients suffer specific and rapid loss of RGCs, predominantly in young adult males, due to complex-I mutations in the mitochondrial genome. This study directly compares the degree of OXPHOS impairment in POAG and LHON patients, testing the hypothesis that the milder clinical disease in POAG is due to a milder complex-I impairment. To assess overall mitochondrial capacity, cells can be forced to produce ATP primarily from mitochondrial OXPHOS by switching the media carbon source to galactose. Under these conditions POAG lymphoblasts grew 1.47 times slower than controls, whilst LHON lymphoblasts demonstrated a greater degree of growth impairment (2.35 times slower). Complex-I enzyme specific activity was reduced by 18% in POAG lymphoblasts and by 29% in LHON lymphoblasts. We also assessed complex-I ATP synthesis, which was 19% decreased in POAG patients and 17% decreased in LHON patients. This study demonstrates both POAG and LHON lymphoblasts have impaired complex-I, and in the majority of aspects the functional defects in POAG were milder than LHON, which could reflect the milder disease development of POAG. This new evidence places POAG in the spectrum of mitochondrial optic neuropathies and raises the possibility for new therapeutic targets aimed at improving mitochondrial function.
Conflict of interest statement
Figures

Similar articles
-
Impaired complex-I-linked respiration and ATP synthesis in primary open-angle glaucoma patient lymphoblasts.Invest Ophthalmol Vis Sci. 2012 Apr 30;53(4):2431-7. doi: 10.1167/iovs.12-9596. Invest Ophthalmol Vis Sci. 2012. PMID: 22427588
-
Increasing mtDNA levels as therapy for mitochondrial optic neuropathies.Drug Discov Today. 2018 Mar;23(3):493-498. doi: 10.1016/j.drudis.2018.01.031. Epub 2018 Jan 11. Drug Discov Today. 2018. PMID: 29337205
-
Mitochondrial abnormalities in patients with primary open-angle glaucoma.Invest Ophthalmol Vis Sci. 2006 Jun;47(6):2533-41. doi: 10.1167/iovs.05-1639. Invest Ophthalmol Vis Sci. 2006. PMID: 16723467
-
Emerging Mitochondrial Therapeutic Targets in Optic Neuropathies.Pharmacol Ther. 2016 Sep;165:132-52. doi: 10.1016/j.pharmthera.2016.06.004. Epub 2016 Jun 8. Pharmacol Ther. 2016. PMID: 27288727 Review.
-
[DNA diagnosis in the age of individual made-to-order medications].Nippon Ganka Gakkai Zasshi. 2004 Dec;108(12):863-85; discussion 886. Nippon Ganka Gakkai Zasshi. 2004. PMID: 15656090 Review. Japanese.
Cited by
-
Glial Cells in Glaucoma: Friends, Foes, and Potential Therapeutic Targets.Front Neurol. 2021 Mar 16;12:624983. doi: 10.3389/fneur.2021.624983. eCollection 2021. Front Neurol. 2021. PMID: 33796062 Free PMC article. Review.
-
The relationship between glutathione levels in leukocytes and ocular clinical parameters in glaucoma.PLoS One. 2019 Dec 30;14(12):e0227078. doi: 10.1371/journal.pone.0227078. eCollection 2019. PLoS One. 2019. PMID: 31887133 Free PMC article.
-
Exploring the Novel Susceptibility Gene Variants for Primary Open-Angle Glaucoma in East Asian Cohorts: The GLAU-GENDISK Study.Sci Rep. 2020 Jan 14;10(1):221. doi: 10.1038/s41598-019-57066-7. Sci Rep. 2020. PMID: 31937794 Free PMC article.
-
Peripheral blood mononuclear cell respiratory function is associated with progressive glaucomatous vision loss.Nat Med. 2024 Aug;30(8):2362-2370. doi: 10.1038/s41591-024-03068-6. Epub 2024 Jun 17. Nat Med. 2024. PMID: 38886621 Free PMC article.
-
Targeting Diet and Exercise for Neuroprotection and Neurorecovery in Glaucoma.Cells. 2021 Feb 1;10(2):295. doi: 10.3390/cells10020295. Cells. 2021. PMID: 33535578 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

