Locus coeruleus neuronal activity determines proclivity to consume alcohol in a selectively-bred line of rats that readily consumes alcohol
- PMID: 26496795
- PMCID: PMC4866802
- DOI: 10.1016/j.alcohol.2015.08.008
Locus coeruleus neuronal activity determines proclivity to consume alcohol in a selectively-bred line of rats that readily consumes alcohol
Abstract
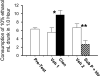
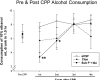
Sprague-Dawley rats selectively-bred for susceptibility to stress in our laboratory (Susceptible, or SUS rats) voluntarily consume large amounts of alcohol, and amounts that have, as shown here, pharmacological effects, which normal rats will not do. In this paper, we explore neural events in the brain that underlie this propensity to readily consume alcohol. Activity of locus coeruleus neurons (LC), the major noradrenergic cell body concentration in the brain, influences firing of ventral tegmentum dopaminergic cell bodies of the mesocorticolimbic system (VTA-DA neurons), which mediate rewarding aspects of alcohol. We tested the hypothesis that in SUS rats alcohol potently suppresses LC activity to markedly diminish LC-mediated inhibition of VTA-DA neurons, which permits alcohol to greatly increase VTA-DA activity and rewarding aspects of alcohol. Electrophysiological single-unit recording of LC and VTA-DA activity showed that in SUS rats alcohol decreased LC burst firing much more than in normal rats and as a result markedly increased VTA-DA activity in SUS rats while having no such effect in normal rats. Consistent with this, in a behavioral test for reward using conditioned place preference (CPP), SUS rats showed alcohol, given by intraperitoneal (i.p.) injection, to be rewarding. Next, manipulation of LC activity by microinfusion of drugs into the LC region of SUS rats showed that (a) decreasing LC activity increased alcohol intake and increasing LC activity decreased alcohol intake in accord with the formulation described above, and (b) increasing LC activity blocked both the rewarding effect of alcohol in the CPP test and the usual alcohol-induced increase in VTA-DA single-unit activity seen in SUS rats. An important ancillary finding in the CPP test was that an increase in LC activity was rewarding by itself, while a decrease in LC activity was aversive; consequently, effects of LC manipulations on alcohol-related reward in the CPP test were perhaps even larger than evident in the test. Finally, when increased LC activity was associated with (i.e., conditioned to) i.p. alcohol, subsequent alcohol consumption by SUS rats was markedly reduced, indicating that SUS rats consume large amounts of alcohol because of rewarding physiological consequences requiring increased VTA-DA activity. The findings reported here are consistent with the view that the influence of alcohol on LC activity leading to changes in VTA-DA activity strongly affects alcohol-mediated reward, and may well be the basis of the proclivity of SUS rats to avidly consume alcohol.
Keywords: Alcohol; Conditioned place preference; Dopamine; Locus coeruleus; Selectively-bred rats; Ventral tegmentum.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
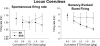
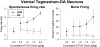
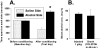


Republished in
-
Reprint of: Locus coeruleus neuronal activity determines proclivity to consume alcohol in a selectively-bred line of rats that readily consumes alcohol.Alcohol. 2016 Feb;50:91-105. doi: 10.1016/j.alcohol.2016.01.001. Alcohol. 2016. PMID: 26873226
Similar articles
-
Reprint of: Locus coeruleus neuronal activity determines proclivity to consume alcohol in a selectively-bred line of rats that readily consumes alcohol.Alcohol. 2016 Feb;50:91-105. doi: 10.1016/j.alcohol.2016.01.001. Alcohol. 2016. PMID: 26873226
-
Noradrenergic inputs from locus coeruleus to posterior ventral tegmental area are essential to support ethanol reinforcement.Addict Biol. 2017 Mar;22(2):291-302. doi: 10.1111/adb.12321. Epub 2015 Nov 8. Addict Biol. 2017. PMID: 26549324
-
Comparison of VTA dopamine neuron activity in lines of rats selectively bred to prefer or avoid alcohol.Alcohol Clin Exp Res. 2006 Jun;30(6):991-7. doi: 10.1111/j.1530-0277.2006.00113.x. Alcohol Clin Exp Res. 2006. PMID: 16737457
-
Testing the hypothesis that locus coeruleus hyperactivity produces depression-related changes via galanin.Neuropeptides. 2005 Jun;39(3):281-7. doi: 10.1016/j.npep.2004.12.028. Epub 2005 Feb 16. Neuropeptides. 2005. PMID: 15944023 Review.
-
The long pursued Holy Grail of the true "alcoholic" rat.Brain Res. 2016 Aug 15;1645:55-7. doi: 10.1016/j.brainres.2016.02.003. Epub 2016 Feb 8. Brain Res. 2016. PMID: 26867703 Review.
Cited by
-
Noradrenergic Mechanisms and Circuitry of Hyperkatifeia in Alcohol Use Disorder.Biol Psychiatry. 2025 Mar 15;97(6):580-589. doi: 10.1016/j.biopsych.2024.09.009. Epub 2024 Sep 18. Biol Psychiatry. 2025. PMID: 39304172 Review.
-
Intravenous Ethanol Administration and Operant Self-Administration Alter Extracellular Norepinephrine Concentration in the Mesocorticolimbic Systems of Male Long Evans Rats.Alcohol Clin Exp Res. 2020 Aug;44(8):1529-1539. doi: 10.1111/acer.14397. Epub 2020 Jul 20. Alcohol Clin Exp Res. 2020. PMID: 32573991 Free PMC article.
-
Lateral hypothalamus-projecting noradrenergic locus coeruleus pathway modulates binge-like ethanol drinking in male and female TH-ires-cre mice.Neuropharmacology. 2021 Sep 15;196:108702. doi: 10.1016/j.neuropharm.2021.108702. Epub 2021 Jul 8. Neuropharmacology. 2021. PMID: 34246685 Free PMC article.
-
Age-dependent dysregulation of locus coeruleus firing in a transgenic rat model of Alzheimer's disease.Neurobiol Aging. 2023 May;125:98-108. doi: 10.1016/j.neurobiolaging.2023.01.016. Epub 2023 Feb 1. Neurobiol Aging. 2023. PMID: 36889122 Free PMC article.
References
-
- Andén N, Grabowska M. Pharmacological evidence for a stimulation of dopamine neurons by noradrenaline neurons in the brain. European Journal of Pharmacology. 1976;39:275–282. - PubMed
-
- Aston Jones G, Foote SL, Bloom FE. Low doses of ethanol disrupt sensory responses of brain noradrenergic neurones. Nature. 1982;296:857–860. - PubMed
-
- Bartfai T, Iverfeldt K, Fisone G, Serfözö P. Regulation of the release of coexisting neurotransmitters. Annual Review of Pharmacology and Toxicology. 1988;28:285–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

