Iron and zinc exploitation during bacterial pathogenesis
- PMID: 26497057
- PMCID: PMC4836889
- DOI: 10.1039/c5mt00170f
Iron and zinc exploitation during bacterial pathogenesis
Abstract
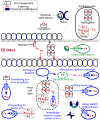
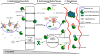
Ancient bacteria originated from metal-rich environments. Billions of years of evolution directed these tiny single cell creatures to exploit the versatile properties of metals in catalyzing chemical reactions and biological responses. The result is an entire metallome of proteins that use metal co-factors to facilitate key cellular process that range from the production of energy to the replication of DNA. Two key metals in this regard are iron and zinc, both abundant on Earth but not readily accessible in a human host. Instead, pathogenic bacteria must employ clever ways to acquire these metals. In this review we describe the many elegant ways these bacteria mine, regulate, and craft the use of two key metals (iron and zinc) to build a virulence arsenal that challenges even the most sophisticated immune response.
Figures
References
-
- Wicander R, Monroe J. Essentials of geology. 4th. Cengage Learning; Boston, MA: 2005. pp. 63–64.
-
- Vallee B, Falchuk H. Physiol Rev. 1993;73:79–118. - PubMed
-
- Andreini C, Banci L, Bertini I, Rosato A. J Proteome Res. 2006;5:3173–3178. - PubMed
-
- Kappler A, Straub KL. Rev Mineral Geochemistry. 2005;59:85–108.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

