EZH2 in normal hematopoiesis and hematological malignancies
- PMID: 26497210
- PMCID: PMC4823035
- DOI: 10.18632/oncotarget.6198
EZH2 in normal hematopoiesis and hematological malignancies
Abstract
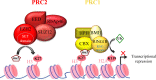
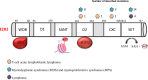
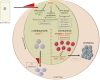
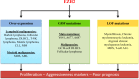
Enhancer of zeste homolog 2 (EZH2), the catalytic subunit of the Polycomb repressive complex 2, inhibits gene expression through methylation on lysine 27 of histone H3. EZH2 regulates normal hematopoietic stem cell self-renewal and differentiation. EZH2 also controls normal B cell differentiation. EZH2 deregulation has been described in many cancer types including hematological malignancies. Specific small molecules have been recently developed to exploit the oncogenic addiction of tumor cells to EZH2. Their therapeutic potential is currently under evaluation. This review summarizes the roles of EZH2 in normal and pathologic hematological processes and recent advances in the development of EZH2 inhibitors for the personalized treatment of patients with hematological malignancies.
Keywords: EZH2; Polycomb complex; hematological malignancies; therapeutic target.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
[EZH2 is therapeutic target for personalized treatment in multiple myeloma].Bull Cancer. 2018 Sep;105(9):804-819. doi: 10.1016/j.bulcan.2018.06.003. Epub 2018 Jul 2. Bull Cancer. 2018. PMID: 30041976 Review. French.
-
EZH2 in normal and malignant hematopoiesis.Leukemia. 2014 Jan;28(1):44-9. doi: 10.1038/leu.2013.288. Epub 2013 Oct 7. Leukemia. 2014. PMID: 24097338 Review.
-
Ezh2 loss in hematopoietic stem cells predisposes mice to develop heterogeneous malignancies in an Ezh1-dependent manner.Blood. 2015 Sep 3;126(10):1172-83. doi: 10.1182/blood-2015-03-634428. Epub 2015 Jul 28. Blood. 2015. PMID: 26219303
-
Selective inhibition of EZH2 by ZLD1039 blocks H3K27 methylation and leads to potent anti-tumor activity in breast cancer.Sci Rep. 2016 Feb 12;6:20864. doi: 10.1038/srep20864. Sci Rep. 2016. PMID: 26868841 Free PMC article.
-
Multifaceted role of the polycomb-group gene EZH2 in hematological malignancies.Int J Hematol. 2017 Jan;105(1):23-30. doi: 10.1007/s12185-016-2124-x. Epub 2016 Nov 9. Int J Hematol. 2017. PMID: 27830540 Review.
Cited by
-
Molecular complexity of diffuse large B-cell lymphoma: a molecular perspective and therapeutic implications.J Appl Genet. 2024 Feb;65(1):57-72. doi: 10.1007/s13353-023-00804-5. Epub 2023 Nov 25. J Appl Genet. 2024. PMID: 38001281 Review.
-
PRC2 targeting is a therapeutic strategy for EZ score defined high-risk multiple myeloma patients and overcome resistance to IMiDs.Clin Epigenetics. 2018 Oct 3;10(1):121. doi: 10.1186/s13148-018-0554-4. Clin Epigenetics. 2018. PMID: 30285865 Free PMC article.
-
Metformin's Modulatory Effects on miRNAs Function in Cancer Stem Cells-A Systematic Review.Cells. 2020 Jun 4;9(6):1401. doi: 10.3390/cells9061401. Cells. 2020. PMID: 32512882 Free PMC article.
-
Functional and therapeutic significance of EZH2 in urological cancers.Oncotarget. 2017 Jun 6;8(23):38044-38055. doi: 10.18632/oncotarget.16765. Oncotarget. 2017. PMID: 28410242 Free PMC article. Review.
-
Effect of Rifampicin on the Pharmacokinetics of Valemetostat and Its Primary Metabolite: A Phase 1 Study in Healthy Participants.Clin Transl Sci. 2025 Jul;18(7):e70279. doi: 10.1111/cts.70279. Clin Transl Sci. 2025. PMID: 40622841 Free PMC article. Clinical Trial.
References
-
- Shaknovich R, De S, Michor F. Epigenetic diversity in hematopoietic neoplasms. Biochim Biophys Acta. 2014;1846:477–484. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

