Fc receptors in antibody-dependent enhancement of viral infections
- PMID: 26497532
- PMCID: PMC7165974
- DOI: 10.1111/imr.12367
Fc receptors in antibody-dependent enhancement of viral infections
Abstract
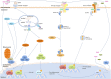
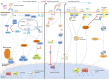
Sensitization of the humoral immune response to invading viruses and production of antiviral antibodies forms part of the host antiviral repertoire. Paradoxically, for a number of viral pathogens, under certain conditions, antibodies provide an attractive means of enhanced virus entry and replication in a number of cell types. Known as antibody-dependent enhancement (ADE) of infection, the phenomenon occurs when virus-antibody immunocomplexes interact with cells bearing complement or Fc receptors, promoting internalization of the virus and increasing infection. Frequently associated with exacerbation of viral disease, ADE of infection presents a major obstacle to the prevention of viral disease by vaccination and is thought to be partly responsible for the adverse effects of novel antiviral therapeutics such as intravenous immunoglobulins. There is a growing body of work examining the intracellular signaling pathways and epitopes responsible for mediating ADE, with a view to aiding rational design of antiviral strategies. With in vitro studies also confirming ADE as a feature of infection for a growing number of viruses, challenges remain in understanding the multilayered molecular mechanisms of ADE and its effect on viral pathogenesis.
Keywords: Fc receptors; antibody-dependent enhancement; intravenous immunoglobulins; virus.
© 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
References
-
- Klasse PJ, Sattentau QJ. Occupancy and mechanism in antibody‐mediated neutralization of animal viruses. J Gen Virol 2002;83:2091–2108. - PubMed
-
- Hawkes RA. Enhancement of the infectivity of arboviruses by specific antisera produced in domestic fowls. Aust J Exp Biol Med Sci 1964;42:465–482. - PubMed
-
- Hawkes RA, Lafferty KJ. Enhancement of virus infectivity by antibody. Virology 1967;33:250–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

