Association of activated Gαq to the tumor suppressor Fhit is enhanced by phospholipase Cβ
- PMID: 26497576
- PMCID: PMC4619496
- DOI: 10.1186/s12885-015-1802-z
Association of activated Gαq to the tumor suppressor Fhit is enhanced by phospholipase Cβ
Abstract
Background: G proteins are known to modulate various growth signals and are implicated in the regulation of tumorigenesis. The tumor suppressor Fhit is a newly identified interaction partner of Gq proteins that typically stimulate the phospholipase C pathway. Activated Gαq subunits have been shown to interact directly with Fhit, up-regulate Fhit expression and enhance its suppressive effect on cell growth and migration. Other signaling molecules may be involved in modulating Gαq/Fhit interaction.
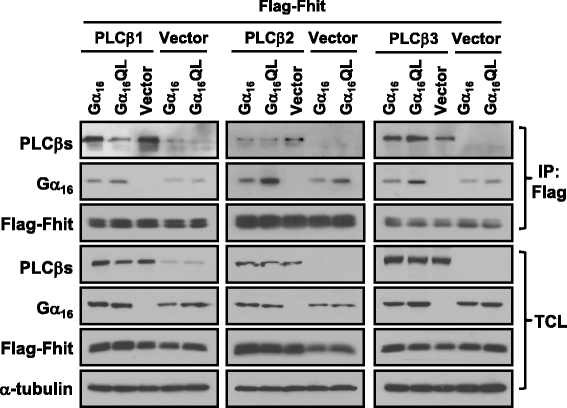
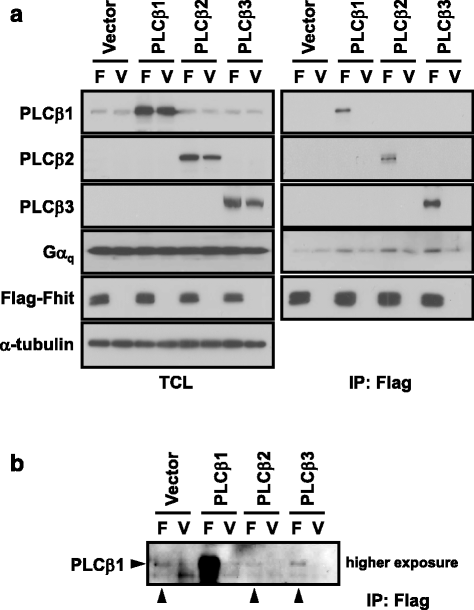
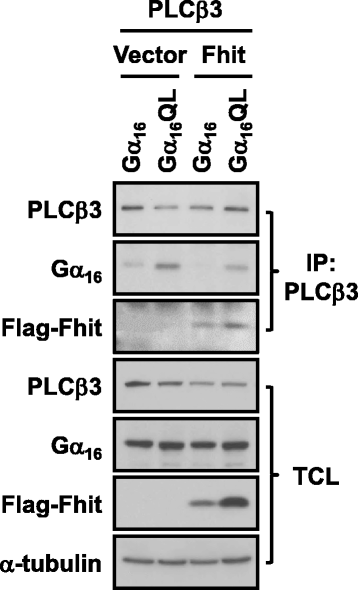
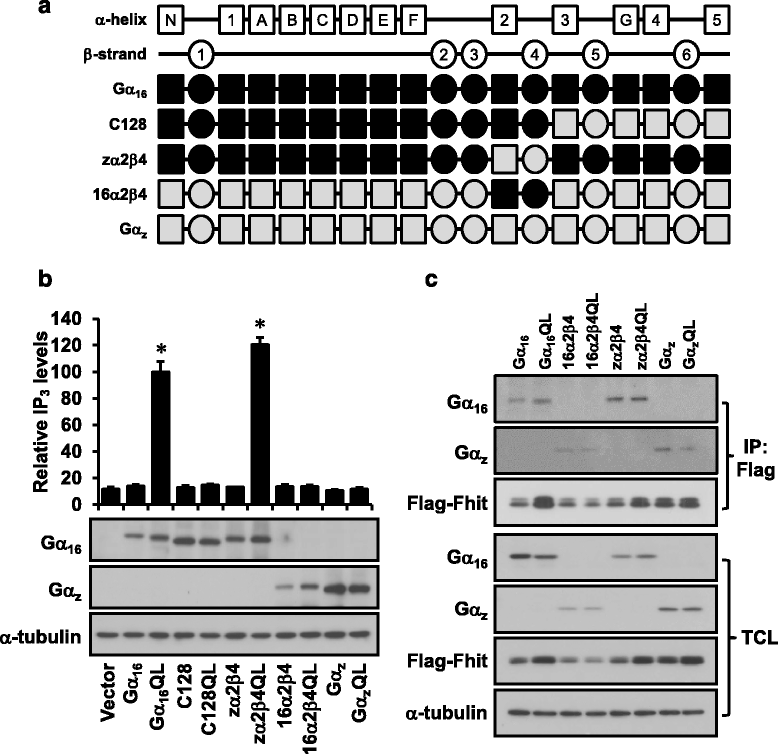
Methods: To test the relationship of PLCβ with the interaction between Gαq and Fhit, co-immunoprecipication assay was performed on HEK293 cells co-transfected with different combinations of Flag-Fhit, Gα16, Gα16QL, pcDNA3 vector, and PLCβ isoforms. Possible associations of Fhit with other effectors of Gαq were also demonstrated by co-immunoprecipitation. The regions of Gαq for Fhit interaction and PLCβ stimulation were further evaluated by inositol phosphates accumulation assay using a series of Gα16/z chimeras with discrete regions of Gα16 replaced by those of Gαz.
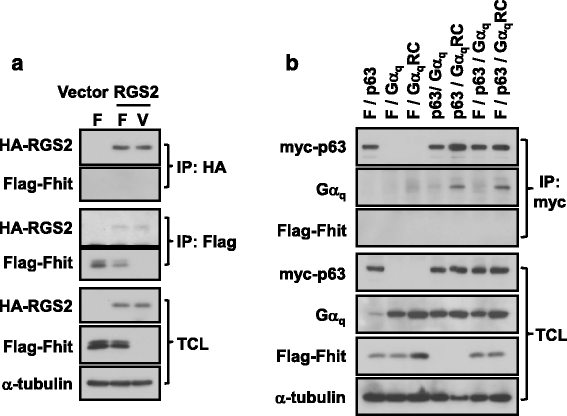
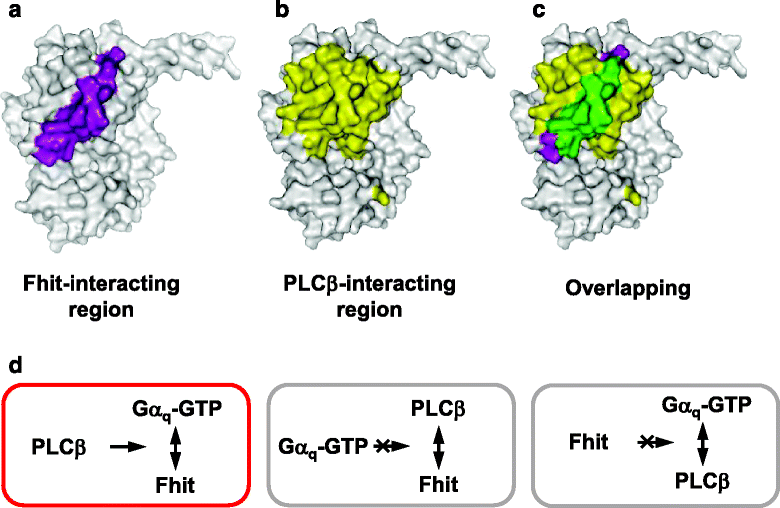
Results: PLCβ1, 2 and 3 interacted with Fhit regardless of the expression of Gαq. Expression of PLCβ increased the affinities of Fhit for both wild-type and activated Gαq. Swapping of the Fhit-interacting α2-β4 region of Gαq with Gαi eliminated the association of Gαq with Fhit without affecting the ability of the mutant to stimulate PLCβ. Other effectors of Gαq including RGS2 and p63RhoGEF were unable to interact with Fhit.
Conclusions: PLCβ may participate in the regulation of Fhit by Gq in a unique way. PLCβ interacts with Fhit and increases the interaction between Gαq and Fhit. The Gαq/PLCβ/Fhit complex formation points to a novel signaling pathway that may negatively regulate tumor cell growth.
Figures
Similar articles
-
Activation state-dependent interaction between Gαq subunits and the Fhit tumor suppressor.Cell Commun Signal. 2013 Aug 15;11:59. doi: 10.1186/1478-811X-11-59. Cell Commun Signal. 2013. PMID: 23947369 Free PMC article.
-
Activation of Gαq subunits up-regulates the expression of the tumor suppressor Fhit.Cell Signal. 2013 Dec;25(12):2440-52. doi: 10.1016/j.cellsig.2013.08.019. Epub 2013 Aug 30. Cell Signal. 2013. PMID: 23993961
-
An intact helical domain is required for Gα14 to stimulate phospholipase Cβ.BMC Struct Biol. 2015 Sep 16;15:18. doi: 10.1186/s12900-015-0043-3. BMC Struct Biol. 2015. PMID: 26377666 Free PMC article.
-
Phospholipase Cβ connects G protein signaling with RNA interference.Adv Biol Regul. 2016 May;61:51-7. doi: 10.1016/j.jbior.2015.11.006. Epub 2015 Dec 2. Adv Biol Regul. 2016. PMID: 26746047 Free PMC article. Review.
-
Gαq signalling: the new and the old.Cell Signal. 2014 May;26(5):833-48. doi: 10.1016/j.cellsig.2014.01.010. Epub 2014 Jan 17. Cell Signal. 2014. PMID: 24440667 Review.
Cited by
-
The nuclear receptor ERβ engages AGO2 in regulation of gene transcription, RNA splicing and RISC loading.Genome Biol. 2017 Oct 6;18(1):189. doi: 10.1186/s13059-017-1321-0. Genome Biol. 2017. PMID: 29017520 Free PMC article.
References
-
- Ji L, Fang B, Yen N, Fong K, Minna JD, Roth JA. Induction of apoptosis and inhibition of tumorigenicity and tumor growth by adenovirus vector-mediated fragile histidine triad (FHIT) gene overexpression. Cancer Res. 1999;59(14):3333–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

