SERPINB12 Is a Slow-Binding Inhibitor of Granzyme A and Hepsin
- PMID: 26497600
- PMCID: PMC4900762
- DOI: 10.1021/acs.biochem.5b01042
SERPINB12 Is a Slow-Binding Inhibitor of Granzyme A and Hepsin
Abstract
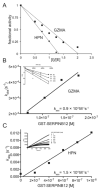
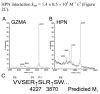
The clade B/intracellular serpins protect cells from peptidase-mediated injury by forming covalent complexes with their targets. SERPINB12 is expressed in most tissues, especially at cellular interfaces with the external environment. This wide tissue distribution pattern is similar to that of granzyme A (GZMA). Because SERPINB12 inhibits trypsin-like serine peptidases, we determined whether it might also neutralize GZMA. SERPINB12 formed a covalent complex with GZMA and inhibited the enzyme with typical serpin slow-binding kinetics. SERPINB12 also inhibited Hepsin. SERPINB12 may function as an endogenous inhibitor of these peptidases.
Figures

Similar articles
-
SERPINB12 is a novel member of the human ov-serpin family that is widely expressed and inhibits trypsin-like serine proteinases.J Biol Chem. 2001 Dec 28;276(52):49320-30. doi: 10.1074/jbc.M108879200. Epub 2001 Oct 16. J Biol Chem. 2001. PMID: 11604408
-
Human SERPINB12 Is an Abundant Intracellular Serpin Expressed in Most Surface and Glandular Epithelia.J Histochem Cytochem. 2015 Nov;63(11):854-65. doi: 10.1369/0022155415600498. Epub 2015 Jul 28. J Histochem Cytochem. 2015. PMID: 26220980 Free PMC article.
-
Serine and cysteine proteases are translocated to similar extents upon formation of covalent complexes with serpins. Fluorescence perturbation and fluorescence resonance energy transfer mapping of the protease binding site in CrmA complexes with granzyme B and caspase-1.J Biol Chem. 2007 Jan 26;282(4):2305-13. doi: 10.1074/jbc.M609546200. Epub 2006 Dec 1. J Biol Chem. 2007. PMID: 17142451
-
Control of granzymes by serpins.Cell Death Differ. 2010 Apr;17(4):586-95. doi: 10.1038/cdd.2009.169. Epub 2009 Nov 6. Cell Death Differ. 2010. PMID: 19893573 Review.
-
Delivery and therapeutic potential of human granzyme B.Immunol Rev. 2010 May;235(1):159-71. doi: 10.1111/j.0105-2896.2010.00894.x. Immunol Rev. 2010. PMID: 20536562 Review.
Cited by
-
Molecular characterization of serine protease inhibitor isoform 3, SmSPI, from Schistosoma mansoni.Parasitol Res. 2016 Aug;115(8):2981-94. doi: 10.1007/s00436-016-5053-y. Epub 2016 Apr 16. Parasitol Res. 2016. PMID: 27083187
-
Decoding the mechanisms of chimeric antigen receptor (CAR) T cell-mediated killing of tumors: insights from granzyme and Fas inhibition.Cell Death Dis. 2024 Feb 2;15(2):109. doi: 10.1038/s41419-024-06461-8. Cell Death Dis. 2024. PMID: 38307835 Free PMC article.
-
Optimization of differentiation and transcriptomic profile of THP-1 cells into macrophage by PMA.PLoS One. 2023 Jul 17;18(7):e0286056. doi: 10.1371/journal.pone.0286056. eCollection 2023. PLoS One. 2023. PMID: 37459313 Free PMC article.
-
Neurobiochemical, Peptidomic, and Bioinformatic Approaches to Characterize Tauopathy Peptidome Biomarker Candidates in Experimental Mouse Model of Traumatic Brain Injury.Mol Neurobiol. 2023 Apr;60(4):2295-2319. doi: 10.1007/s12035-022-03165-y. Epub 2023 Jan 13. Mol Neurobiol. 2023. PMID: 36635478
-
Skin tape proteomics identifies pathways associated with transepidermal water loss and allergen polysensitization in atopic dermatitis.J Allergy Clin Immunol. 2020 Dec;146(6):1367-1378. doi: 10.1016/j.jaci.2020.04.022. Epub 2020 Apr 28. J Allergy Clin Immunol. 2020. PMID: 32360271 Free PMC article. Clinical Trial.
References
-
- Askew YS, Pak SC, Luke CJ, Askew DJ, Cataltepe S, Mills DR, Kato H, Lehoczky J, Dewar K, Birren B, Silverman GA. SERPINB12 is a novel member of the human ov-serpin family that is widely expressed and inhibits trypsin-like serine proteinases. J Biol Chem. 2001;276:49320–49330. - PubMed
-
- Silverman GA, Whisstock JC, Askew DJ, Pak SC, Luke CJ, Cataltepe S, Irving JA, Bird PI. Human clade B serpins (ov-serpins) belong to a cohort of evolutionarily dispersed intracellular proteinase inhibitor clades that protect cells from promiscuous proteolysis. Cell Mol Life Sci. 2004;61:301–325. - PMC - PubMed
-
- Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000;407:923–926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

