A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes
- PMID: 26497614
- PMCID: PMC4988239
- DOI: 10.1016/j.yjmcc.2015.10.022
A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes
Abstract
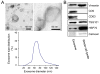
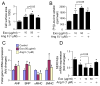
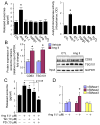
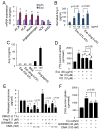
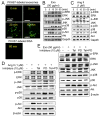
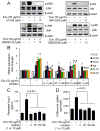
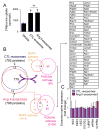
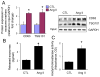
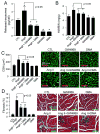
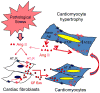
Chronic activation of the myocardial renin angiotensin system (RAS) elevates the local level of angiotensin II (Ang II) thereby inducing pathological cardiac hypertrophy, which contributes to heart failure. However, the precise underlying mechanisms have not been fully delineated. Herein we report a novel paracrine mechanism between cardiac fibroblasts (CF)s and cardiomyocytes whereby Ang II induces pathological cardiac hypertrophy. In cultured CFs, Ang II treatment enhanced exosome release via the activation of Ang II receptor types 1 (AT1R) and 2 (AT2R), whereas lipopolysaccharide, insulin, endothelin (ET)-1, transforming growth factor beta (TGFβ)1 or hydrogen peroxide did not. The CF-derived exosomes upregulated the expression of renin, angiotensinogen, AT1R, and AT2R, downregulated angiotensin-converting enzyme 2, and enhanced Ang II production in cultured cardiomyocytes. In addition, the CF exosome-induced cardiomyocyte hypertrophy was blocked by both AT1R and AT2R antagonists. Exosome inhibitors, GW4869 and dimethyl amiloride (DMA), inhibited CF-induced cardiomyocyte hypertrophy with little effect on Ang II-induced cardiomyocyte hypertrophy. Mechanistically, CF exosomes upregulated RAS in cardiomyocytes via the activation of mitogen-activated protein kinases (MAPKs) and Akt. Finally, Ang II-induced exosome release from cardiac fibroblasts and pathological cardiac hypertrophy were dramatically inhibited by GW4869 and DMA in mice. These findings demonstrate that Ang II stimulates CFs to release exosomes, which in turn increase Ang II production and its receptor expression in cardiomyocytes, thereby intensifying Ang II-induced pathological cardiac hypertrophy. Accordingly, specific targeting of Ang II-induced exosome release from CFs may serve as a novel therapeutic approach to treat cardiac pathological hypertrophy and heart failure.
Keywords: Angiotensin II; Cardiac hypertrophy; Exosomes; Heart failure.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Klotho inhibits angiotensin II-induced cardiomyocyte hypertrophy through suppression of the AT1R/beta catenin pathway.Biochem Biophys Res Commun. 2016 Apr 29;473(2):455-61. doi: 10.1016/j.bbrc.2016.03.029. Epub 2016 Mar 10. Biochem Biophys Res Commun. 2016. PMID: 26970306
-
Adenovirus-mediated overexpression and stimulation of the human angiotensin II type 2 receptor in porcine cardiac fibroblasts does not modulate proliferation, collagen I mRNA expression and ERK1/ERK2 activity, but inhibits protein tyrosine phosphatases.J Mol Med (Berl). 2001 Sep;79(9):510-21. doi: 10.1007/s001090100243. J Mol Med (Berl). 2001. PMID: 11692164
-
Exosomes derived from cardiac fibroblasts with Ang-II stimulation provoke myocardial hypertrophy via miR-15b-5p/PTEN-L axis.Exp Cell Res. 2025 Jan 15;444(2):114380. doi: 10.1016/j.yexcr.2024.114380. Epub 2024 Dec 12. Exp Cell Res. 2025. PMID: 39674360
-
Role of the renin-angiotensin system in cardiac hypertrophy.Am J Cardiol. 1999 Jun 17;83(12A):53H-57H. doi: 10.1016/s0002-9149(99)00259-3. Am J Cardiol. 1999. PMID: 10750588 Review.
-
The renin-angiotensin system and experimental heart failure.Cardiovasc Res. 1999 Sep;43(4):838-49. doi: 10.1016/s0008-6363(99)00145-5. Cardiovasc Res. 1999. PMID: 10615411 Review.
Cited by
-
Attenuation of Experimental Autoimmune Hepatitis in Mice with Bone Mesenchymal Stem Cell-Derived Exosomes Carrying MicroRNA-223-3p.Mol Cells. 2019 Dec 31;42(12):906-918. doi: 10.14348/molcells.2019.2283. Mol Cells. 2019. PMID: 31826604 Free PMC article.
-
Extracellular vesicles in cardiovascular disease: Biological functions and therapeutic implications.Pharmacol Ther. 2022 May;233:108025. doi: 10.1016/j.pharmthera.2021.108025. Epub 2021 Oct 20. Pharmacol Ther. 2022. PMID: 34687770 Free PMC article. Review.
-
Roles of Exosomes in Cardiac Fibroblast Activation and Fibrosis.Cells. 2021 Oct 28;10(11):2933. doi: 10.3390/cells10112933. Cells. 2021. PMID: 34831158 Free PMC article. Review.
-
Stem Cell-Derived Extracellular Vesicles in the Treatment of Cardiovascular Diseases.Pharmaceutics. 2024 Mar 11;16(3):381. doi: 10.3390/pharmaceutics16030381. Pharmaceutics. 2024. PMID: 38543275 Free PMC article. Review.
-
Competitive signaling and cellular communications in myocardial infarction response.Mol Biol Rep. 2025 Jan 16;52(1):129. doi: 10.1007/s11033-025-10236-5. Mol Biol Rep. 2025. PMID: 39820809 Free PMC article. Review.
References
-
- Wollert KC, Drexler H. The renin-angiotensin system and experimental heart failure. Cardiovascular research. 1999;43:838–49. - PubMed
-
- Balakumar P, Jagadeesh G. A century old renin-angiotensin system still grows with endless possibilities: AT1 receptor signaling cascades in cardiovascular physiopathology. Cell Signal. 2014;26:2147–60. - PubMed
-
- Gul R, Shawl AI, Kim SH, Kim UH. Cooperative interaction between reactive oxygen species and Ca2+ signals contributes to angiotensin II-induced hypertrophy in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2012;302:H901–9. - PubMed
-
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. Journal of proteomics. 2010;73:1907–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

