Recurrent multiple CNS hemangioblastomas with VHL disease treated with pazopanib: a case report and literature review
- PMID: 26497655
- PMCID: PMC6083944
- DOI: 10.2217/cns.15.22
Recurrent multiple CNS hemangioblastomas with VHL disease treated with pazopanib: a case report and literature review
Abstract
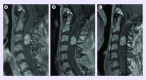
Hemangioblastoma is a rare benign neoplasm, accounting for less than 2% of all primitive brain tumors. It may arise sporadically in a solitary form, or associated with Von Hippel-Lindau (VHL) disease with multiple tumors. Surgery is the mainstay treatment, but management is challenging in case of recurrent and/or multiple tumors. VHL protein is defective in both forms of hemangioblastoma, leading to the accumulation of hypoxia-inducible factor, stimulating angiogenesis via VEGF and PDGF mainly. Here, we report a 37-year-old woman's case with recurrent and rapidly progressive VHL-associated hemangioblastomas, causing severe disability. She was treated 24 months with pazopanib, a multityrosine kinase inhibitor (TKI) targeting VEGF and PDGF-β pathways. Despite moderate radiological changes, progressive improvement in her clinical condition persisting over 3 years was observed. Inhibiting angiogenesis is a therapeutic option that may improve the quality of life and the autonomy of VHL patients disabled with multiple hemangioblastomas.
Keywords: Von Hippel–Lindau disease; hemangioblastoma; pazopanib.
Conflict of interest statement
Figures


References
-
- Kim BY, Jonasch E, Mccutcheon IE. Pazopanib therapy for cerebellar hemangioblastomas in von Hippel–Lindau disease: case report. Target Oncol. 2012;7(2):145–149. - PMC - PubMed
-
•• Report of oral pazopanib resulting in significant neurologic improvement and radiologic tumor volume reduction in CNS hemangioblastomas associated with VHL disease.
-
- Lonser RR, Glenn GM, Walther M, et al. Von Hippel–Lindau disease. Lancet. 2003;361(9374):2059–2067. - PubMed
-
• This article provides an overview of the clinical aspects, management and treatment options for von Hippel–Lindau disease.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
