A putative biomarker signature for clinically effective AKT inhibition: correlation of in vitro, in vivo and clinical data identifies the importance of modulation of the mTORC1 pathway
- PMID: 26497682
- PMCID: PMC4747185
- DOI: 10.18632/oncotarget.6153
A putative biomarker signature for clinically effective AKT inhibition: correlation of in vitro, in vivo and clinical data identifies the importance of modulation of the mTORC1 pathway
Abstract
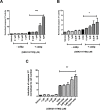
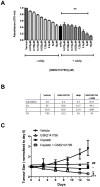
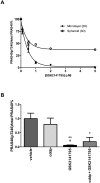
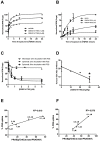
Our identification of dysregulation of the AKT pathway in ovarian cancer as a platinum resistance specific event led to a comprehensive analysis of in vitro, in vivo and clinical behaviour of the AKT inhibitor GSK2141795. Proteomic biomarker signatures correlating with effects of GSK2141795 were developed using in vitro and in vivo models, well characterised for related molecular, phenotypic and imaging endpoints. Signatures were validated in temporally paired biopsies from patients treated with GSK2141795 in a clinical study. GSK2141795 caused growth-arrest as single agent in vitro, enhanced cisplatin-induced apoptosis in vitro and reduced tumour volume in combination with platinum in vivo. GSK2141795 treatment in vitro and in vivo resulted in ~50-90% decrease in phospho-PRAS40 and 20-80% decrease in fluoro-deoxyglucose (FDG) uptake. Proteomic analysis of GSK2141795 in vitro and in vivo identified a signature of pathway inhibition including changes in AKT and p38 phosphorylation and total Bim, IGF1R, AR and YB1 levels. In patient biopsies, prior to treatment with GSK2141795 in a phase 1 clinical trial, this signature was predictive of post-treatment changes in the response marker CA125. Development of this signature represents an opportunity to demonstrate the clinical importance of AKT inhibition for re-sensitisation of platinum resistant ovarian cancer to platinum.
Keywords: AKT; biomarkers; ovarian cancer; platinum resistance; proteomics.
Conflict of interest statement
All employees of GlaxoSmithKline are identified by their affiliations.
Figures

References
-
- Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, De Geest K, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–1425. - PMC - PubMed
-
- Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nature reviews Cancer. 2010;10:803–808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

