Oral budesonide for induction of remission in ulcerative colitis
- PMID: 26497719
- PMCID: PMC9239584
- DOI: 10.1002/14651858.CD007698.pub3
Oral budesonide for induction of remission in ulcerative colitis
Abstract
Background: Corticosteroids are first-line therapy for induction of remission in ulcerative colitis. Although corticosteroids may improve symptoms, they have significant adverse effects. Steroids which act topically, with less systemic side-effects may be more desirable. Budesonide is a topically acting corticosteroid with extensive first pass hepatic metabolism. There are currently three formulations of budesonide: two standard formulations including a controlled-ileal release capsule and a pH-dependent capsule both designed to release the drug in the distal small intestine and right colon; and the newer Budesonide-MMX® capsule designed to release the drug throughout the entire colon.
Objectives: The primary objective was to evaluate the efficacy and safety of oral budesonide for the induction of remission in ulcerative colitis.
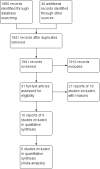
Search methods: We searched MEDLINE, EMBASE, CENTRAL, and the Cochrane IBD Group Specialised Register from inception to April 2015. We also searched reference lists of articles, conference proceedings and ClinicalTrials.gov.
Selection criteria: Randomised controlled trials comparing oral budesonide to placebo or another active therapy for induction of remission in ulcerative colitis were considered eligible. There were no exclusions based on patient age or the type, dose, duration or formulation of budesonide therapy.
Data collection and analysis: Two independent investigators reviewed studies for eligibility, extracted data and assessed study quality. Methodological quality was assessed using the Cochrane risk of bias tool. The overall quality of the evidence supporting the outcomes was evaluated using the GRADE criteria. The primary outcome was induction of remission (as defined by the primary studies) at week eight. Secondary outcomes included clinical, endoscopic and histologic improvement, adverse events and early withdrawal. We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for each dichotomous outcome and the mean difference (MD) and corresponding 95% CI for each continuous outcome. Data were analysed on an intention-to-treat basis.
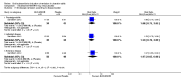
Main results: Six studies (1808 participants) were included. Four studies compared budesonide-MMX® with placebo, one small pilot study looked at clinical remission at week four, and was subsequently followed by three large, studies that assessed combined clinical and endoscopic remission at week eight. Although two placebo-controlled studies had mesalamine and Entocort (standard budesonide) treatment arms, these studies were not sufficiently powered to compare Budesonide-MMX® with these active comparators. One small study compared standard budesonide with prednisolone and one study compared standard budesonide to mesalamine. Four studies were rated as low risk of bias and two studies had an unclear risk of bias. A pooled analysis of three studies (900 participants) showed that budesonide-MMX® 9 mg was significantly superior to placebo for inducing remission (combined clinical and endoscopic remission) at 8 weeks. Fifteen per cent (71/462) of budesonide-MMX® 9 mg patients achieved remission compared to 7% (30/438) of placebo patients (RR 2.25, 95% CI 1.50 to 3.39). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (101 events). A subgroup analysis by concurrent mesalamine use suggests higher efficacy in the 442 patients who were not considered to be mesalamine-refractory (RR 2.89, 95% CI 1.59 to 5.25). A subgroup analysis by disease location suggests budesonide is most effective in patients with left-sided disease (RR 2.98, 95% CI 1.56 to 5.67; 289 patients). A small pilot study reported no statistically significant difference in endoscopic remission between budesonide and prednisolone (RR 0.75, 95% CI 0.23 to 2.42; 72 patients). GRADE indicated that the overall quality of the evidence supporting this outcome was very low due to unclear risk of bias and very sparse data (10 events). Standard oral budesonide was significantly less likely to induce clinical remission than oral mesalamine after 8 weeks of therapy (RR 0.72, 95% CI 0.57 to 0.91; 1 study, 343 patients). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (161 events). Another study found no difference in remission rates between budesonide-MMX® 9 mg and mesalamine (RR 1.48, 95% CI 0.81 to 2.71; 247 patients). GRADE indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (37 events). One study found no difference in remission rates between budesonide-MMX® 9 mg and standard budesonide 9 mg (RR 1.38, 95% CI 0.72 to 2.65; 212 patients). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (32 events). Suppression of plasma cortisol was more common in prednisolone-treated patients (RR 0.02, 95% CI 0.0 to 0.33). While budesonide does appear to suppress morning cortisol to some extent, mean morning cortisol values remained within the normal range in 2 large studies (n = 899) and there was no difference in glucocorticoid-related side-effects across different treatment groups. Further, study withdrawal due to adverse events was not more common in budesonide compared with placebo treated patients (RR 0.85, 95% CI 0.53 to 1.38). Common adverse events included worsening ulcerative colitis, headache, pyrexia, insomnia, back pain, nausea, abdominal pain, diarrhoea, flatulence and nasopharyngitis.
Authors' conclusions: Moderate quality evidence to supports the use of oral budesonide-MMX® at a 9 mg daily dose for induction of remission in active ulcerative colitis, particularly in patients with left-sided colitis. Budesonide-MMX® 9 mg daily is effective for induction of remission in the presence or absence of concurrent 5-ASA therapy. Further, budesonide-MMX® appears to be safe, and does not lead to significant impairment of adrenocorticoid function compared to placebo. Moderate quality evidence from a single study suggests that mesalamine may be superior to standard budesonide for the treatment of active ulcerative colitis. Low quality evidence from one study found no difference in remission rates between budesonide MMX® and mesalamine. Very low quality evidence from one small study showed no difference in endoscopic remission rates between standard budesonide and prednisolone. Low quality evidence from one study showed no difference in remission rates between budesonide-MMX® and standard budesonide. Adequately powered studies are needed to allow conclusions regarding the comparative efficacy and safety of budesonide versus prednisolone, budesonide-MMX® versus standard budesonide and budesonide versus mesalamine.
Conflict of interest statement
MES: Dr. Sherlock has received fees for consultancy from Abbvie Canada for attending an advisory board meeting. All of the fees received are outside the scope of the submitted work.
AMG: Anne Marie Griffiths has received fee(s) from Johnson and Johnson for Board membership; fee(s) from Janssen, Abbvie and Ferring for consultancy; grants or grants pending from Johnson and Johnson and Abbive; lecture fee(s) from: Abbvie and Merck and payment for development of educational presentations from Ferring. All of these activities are outside the submitted work.
AHS: Hillary Steinhart has received fee(s) from Janssen, Abbvie, Shire, Pendopharm, Pfizer, and Takeda for consultancy; and lecture fee(s) from: Janssen, Abbvie, Shire, Warner Chilcott, Aptalis, and Takeda. His institution has received grants or grants pending from Janssen, Abbvie, Pfizer, Amgen, Takeda and Actavis. All of these activities are outside the submitted work.
JKM: None known.
CHS: Dr. Cynthia Seow has served as a consultant and on advisory boards for Janssen Pharmaceuticals, Abbvie, Takeda, Actavis, and Shire. She has a grant through Janssen Pharmaceuticals. Dr. Seow has also provided lectures for Janssen Pharmaceuticals and Warner Chilcott. All of these activities are outside the submitted work.
Figures







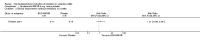



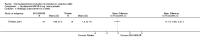























































Update of
-
Oral budesonide for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2010 Oct 6;(10):CD007698. doi: 10.1002/14651858.CD007698.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2015 Oct 26;(10):CD007698. doi: 10.1002/14651858.CD007698.pub3. PMID: 20927762 Updated.
Similar articles
-
Budesonide for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2014 Aug 21;2014(8):CD002913. doi: 10.1002/14651858.CD002913.pub3. Cochrane Database Syst Rev. 2014. PMID: 25141071 Free PMC article.
-
Aminosalicylates for induction of remission or response in Crohn's disease.Cochrane Database Syst Rev. 2016 Jul 3;7(7):CD008870. doi: 10.1002/14651858.CD008870.pub2. Cochrane Database Syst Rev. 2016. PMID: 27372735 Free PMC article.
-
Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2016 Apr 21;4(4):CD000543. doi: 10.1002/14651858.CD000543.pub4. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Aug 12;8:CD000543. doi: 10.1002/14651858.CD000543.pub5. PMID: 27101467 Free PMC article. Updated.
-
Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2012 Oct 17;10:CD000543. doi: 10.1002/14651858.CD000543.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Apr 21;4:CD000543. doi: 10.1002/14651858.CD000543.pub4. PMID: 23076889 Updated.
-
Budesonide for induction of remission in Crohn's disease.Cochrane Database Syst Rev. 2015 Jun 3;2015(6):CD000296. doi: 10.1002/14651858.CD000296.pub4. Cochrane Database Syst Rev. 2015. PMID: 26039678 Free PMC article.
Cited by
-
Budesonide-Loaded Hyaluronic Acid Nanoparticles for Targeted Delivery to the Inflamed Intestinal Mucosa in a Rodent Model of Colitis.Biomed Res Int. 2022 Sep 27;2022:7776092. doi: 10.1155/2022/7776092. eCollection 2022. Biomed Res Int. 2022. PMID: 36203483 Free PMC article.
-
Use, effectiveness and tolerability of budesonide-MMX in ulcerative colitis: A real-life experience.United European Gastroenterol J. 2019 Nov;7(9):1164-1170. doi: 10.1177/2050640619864257. Epub 2019 Jul 17. United European Gastroenterol J. 2019. PMID: 31700629 Free PMC article.
-
A Case of Stevens-Johnson Syndrome Complicated with Multimatrix System Mesalamine in Ulcerative Colitis.Medicina (Kaunas). 2022 Feb 11;58(2):276. doi: 10.3390/medicina58020276. Medicina (Kaunas). 2022. PMID: 35208599 Free PMC article.
-
First United Arab Emirates consensus on diagnosis and management of inflammatory bowel diseases: A 2020 Delphi consensus.World J Gastroenterol. 2020 Nov 21;26(43):6710-6769. doi: 10.3748/wjg.v26.i43.6710. World J Gastroenterol. 2020. PMID: 33268959 Free PMC article.
-
Preliminary evidence in treatment of eosinophilic gastroenteritis in children: A case series.World J Clin Cases. 2022 Jul 6;10(19):6417-6427. doi: 10.12998/wjcc.v10.i19.6417. World J Clin Cases. 2022. PMID: 35979287 Free PMC article.
References
References to studies included in this review
D'Haens 2010 {published and unpublished data}
-
- D'Haens G, Kovács Á, Vergauwe P, Lonovics J, Bouhnik Y, Weiss W, et al. Safety and efficacy of a novel extended release budesonide formulation in patients with active left‐sided ulcerative colitis. Gastroenterology 2007;132(4 Suppl 2):A505‐6.
-
- D'Haens GR, Kovács Á, Vergauwe P, Nagy F, Molnár T, Bouhnik Y, et al. Clinical trial: Preliminary efficacy and safety study of a new budesonide‐MMX® 9 mg extended‐release tablets in patients with active left‐sided ulcerative colitis. Journal of Crohn's and Colitis 2010;4(2):153‐60. - PubMed
Gross 2011 {published and unpublished data}
-
- Gross V, Buganic I, Belousova EA, Mikhailova TL, Kupcinkas L, Kiudelis G, et al. 3g mesalazine granules are superior to 9mg budesonide for achieving remission in active ulcerative colitis: A double‐blind, double‐dummy, randomised trial. Journal of Crohn's and Colitis 2011;5(2):129‐138. - PubMed
-
- Gross V, Bunganic I, Mikhailova TL, Kupcinskas L, Kiudelis G, Tulassay Z, et al. Efficacy and tolerability of a once daily treatment with budesonide capsules versus mesalamine granules for the treatment of active ulcerative colitis: A randomized, double‐blind, double‐dummy, multicenter study. Gastroenterology 2009;136(5 Suppl 1):A15.
Löfberg 1996 {published data only}
-
- Löfberg R, Danielsson Å, Suhr O, Nilsson Å, Schiöler R, Nyberg A, et al. Oral budesonide versus prednisone in patients with active extensive and left‐sided ulcerative colitis. Gastroenterology 1996;110(6):1713‐8. - PubMed
Rubin 2014 {published data only}
-
- Rubin D, Cohen R, Sandborn W, Lichtenstein G, Axler J, Riddell R, et al. Budesonide MMX 9 mg for inducing remission in patients with mild‐to‐moderate ulcerative colitis not adequately controlled with oral 5‐asas. Inflammatory Bowel Diseases 2014;20(Suppl 1):S1.
Sandborn 2012 {published data only}
-
- Sandborn WJ, Travis S, Moro L, Ballard ED, Yeung P, Bleker W, et al. Budesonide Mxx 9mg for the induction of remission of mild‐to‐moderate ulcerative colitis (UC): Data from a multicenter, randomized, double‐blind placebo‐controlled study in North America and India. Gastroenterology 2011;140(5 Suppl 1):S124.
-
- Sandborn WJ, Travis S, Moro L, Jones R, Gautille T, Bagin R, et al. Once‐daily Budesonide MMX® extended‐release tablets induce remission in patients with mild to moderate ulcerative colitis: Results from the CORE 1 study. Gastroenterology 2012;143(5):1218‐26. - PubMed
Travis 2014 {published data only}
-
- Sandborn WJ, Travis S, Danese S, Kupcinskas L, Alexeeva O, Moro L, et al. Budesonide‐MMx 9 mg for induction of remission of mild‐to‐moderate ulcerative colitis (UC): Data from a multicenter, randomized, double‐blind placebo‐controlled study in the Europe, Russia, Israel and Australia. Gastroenterology 2011;140(5 Suppl 1):S65.
References to studies excluded from this review
Chopra 2006 {published data only}
-
- Chopra A, Pardi DS, Loftus EV Jr, Tremaine WJ, Egan LJ, Faubion WA, et al. Budesonide in the treatment of inflammatory bowel disease: the first year of experience in clinical practice. Inflammatory Bowel Diseases 2006;12(1):29‐32. - PubMed
Danese 2013 {published data only}
-
- Danese S, Kupcinskas L, Ballard ED, Harris‐Collazo R, Haridman Y, Huang M, Travis SP. Budesonide MMX® efficacy is independent of previous mesalazine exposure in ulcerative colitis: pooled analysis of CORE I and CORE II. United European Gastroenterology Journal 2013;1(1 Suppl):OP262.
Díaz Blasco 1995 {published data only}
-
- Días Blasco J, Robledo Andrés P. Advances in the topical steroid treatment of inflammatory bowel disease [Avances en el tratamiento esteroideo tópico de la enfermedad inflamatoria intestinal]. Revista Española de Enfermedades Digestivas 1995;87(11):802‐7. - PubMed
Feagan 1996 {published data only}
-
- Feagan B. Budesonide therapy for ulcerative colitis: a topical tale. Gastroenterology 1996;110(6):2000‐17. - PubMed
Fedorak 2005 {published data only}
-
- Fedorak RN, Bistritz L. Targeted delivery, safety, and efficacy of oral enteric‐coated formulations of budesonide. Advanced Drug Delivery Reviews 2005;57(2):303‐16. - PubMed
Gomollón 1999 {published data only}
-
- Gomollón F, Hinojosa J, Nos P. Budesonide and inflammatory bowel disease [Budesonida y enfermedad inflamatoria intestinal]. Gastroenterología y Hepatología 1999;22(10):525‐32. - PubMed
Keller 1997 {published data only}
-
- Keller R, Stoll R, Foerster EC, Gutsche N, Domschke W. Oral budesonide therapy for steroid‐dependent ulcerative colitis. Alimentary Pharmacology and Therapeutics 1997;11(6):1047‐52. - PubMed
Kolkman 2004 {published data only}
-
- Kolkman JJ, Möllmann HW, Möllmann AC, Peńa AS, Greinwald R, Tauschel HD, et al. Evaluation of oral budesonide in the treatment of active distal ulcerative colitis. Drugs of Today 2004;40(7):589‐601. - PubMed
-
- Kolkman JJ, Mölmann HW, Möllman AC, Nelis FG, Viergever P, Peña S. Beneficial effect of oral budesonide for distal ulcerative colitis: A comparative study of Budenfalk® 3 MG TID VS 9 MG OD. Gastroenterology 2000;118(4 Suppl 2):A779.
Lamers 1996 {published data only}
-
- Lamers CB, Wagtmans MJ, Sluys Veer A, Hogezand RA, Griffioen G. Budesonide in inflammatory bowel disease. Netherlands Journal of Medicine 1996;48(2):60‐3. - PubMed
Lichtenstein 2012 {published data only}
-
- Lichtenstein GR, Danese S, Ballard ED, Moro L, Jones RJ, Bagin R, et al. Effect of budesonide MMX 6 mg on the hypothalamic‐pituitary‐adrenal (HPA) axis in patients with ulcerative colitis: Results from a phase III, 12 month safety and extended use study. Gastroenterology 2012;142(5 Suppl 1):S785.
Lichtenstein 2013 {published data only}
-
- Lichtenstein G, Sandborn W, Huang M, Hardiman Y, Bagin R, Yeung P, et al. Budesonide MMX versus placebo in patients with active, mild‐to‐moderate ulcerative colitis who were 5‐ASA naive or previously treated with 5‐ASA. Inflammatory Bowel Diseases 2013;19:S83.
-
- Lichtenstein GR, Sandborn W, Huang M, Hardiman Y, Bagin R, Yeung P, et al. Budesonide MMX 9 mg induces remission in mild‐to‐moderately active UC patients regardless of prior history of 5‐ASA therapy. Gastroenterology 2013;144(5 Suppl 1):S234.
Marín‐Jiménez 2006 {published data only}
-
- Marín‐Jiménez I, Peña AS. Budesonide for ulcerative colitis. Revista Española de Enfermedades Digestivas 2006;98(5):362‐73. - PubMed
Sandborn 2013a {published data only}
-
- Sandborn W, Bagin R, Zhao J, Huang M, Hardiman Y, Schulteis C, et al. Symptom resolution and clinical and endoscopic remission both resulted in improved quality of life in patients with ulcerative colitis. Inflammatory Bowel Diseases 2013;19:S82.
Sandborn 2013b {published data only}
-
- Sandborn W, Hardiman Y, Huang M, Harris‐Collazo R, Ballard ED, Travis S. Efficacy of budesonide MMX in reduction of symptoms in patients with mild‐to‐moderately active ulcerative colitis: A pooled analysis of the core I and core II studies. Gastroenterology 2013;144(5 Suppl 1):S233‐4.
Sandborn 2015 {published data only}
-
- Sandborn W, Travis S, Bala N, Danese S, Moro L, Ballard ED, et al. Induction of clinical and endoscopic remission of mild to moderately active ulcerative colitis with budesonide MMX 9 MG: Analysis of pooled data from two phase 3 studies. American Journal of Gastroenterology 2011;106:S485.
Silverman 2011 {published data only}
-
- Silverman J, Otley A. Budesonide in the treatment of inflammatory bowel disease. Expert Review of Clinical Immunology 2011;7(4):419‐28. - PubMed
Travis 2011 {published data only}
-
- Travis S, Ballard ED II, Bagin B, Gautille T, Huang M. Induction of remission with oral budesonide MMXVR (9 mg) tablets in patients with mild to moderate, active ulcerative colitis: A multicenter, open‐label efficacy and safety study. Inflammatory Bowel Diseases 2011;17(Suppl 2):S20.
Travis 2012b {published data only}
-
- Travis S, Danese S, Moro L, Ballard ED, Bagin R, Gautille T, et al. Induction of clinical and endoscopic remission with budesonide MMX in mild to moderately active ulcerative colitis: Pooled data from two phase 3 studies. Journal of Crohn's and Colitis 2012;6:S74.
Additional references
Bar‐Meir 2003
-
- Bar‐Meir S, Fidder HH, Faszczyk M, Bianchi Porro G, Sturniolo GC, Mickisch O, et al. Budesonide foam vs. hydrocortisone acetate foam in the treatment of active ulcerative proctosigmoiditis. Diseases of the Colon and Rectum 2003;46(7):929‐36. - PubMed
Barnes 2005
-
- Barnes PJ. Molecular mechanisms and cellular effects of glucocorticosteroids. Immunology and allergy clinics of North America 2005;25(3):451‐68. - PubMed
Baumgart 2007
-
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 2007;369(9573):1641‐57. - PubMed
Beattie 1996
-
- Beattie RM, Nicholls SW, Domizio P, Williams CB, Walker‐Smith JA. Endoscopic assessment of the colonic response to corticosteroids in children with ulcerative colitis. Journal of Pediatric Gastroenterology and Nutrition 1996;22(4):373‐9. - PubMed
Biancone 2008
-
- Biancone L, Michetti P, Travis S, Escher J, Moser G, Forbes A, et al. European evidence‐based consensus on the management of ulcerative colitis: special situations. Journal of Crohn's and Colitis 2008;2(1):63‐92. - PubMed
Bickston 2014
Cohen 2000
-
- Cohen RD, Woseth DM, Thisted RA, Hanauer SB. A meta‐analysis and overview of the literature on treatment options for left‐sided ulcerative colitis and ulcerative proctitis. American Journal of Gastroenterology 2000;95(5):1263‐76. - PubMed
Danese 2014
-
- Danese S, Siegel CA, Peyrin‐Biroulet L. Review article: integrating budesonide‐MMX into treatment algorithms for mild‐to‐moderate ulcerative colitis. Alimentary Pharmacology and Therapeutics 2014;39(10):1095‐103. - PubMed
Danielsson 1992
-
- Danielsson A, Löfberg R, Persson T, Salde L, Schiöler R, Suhr O, Willén R. A steroid enema, budesonide, lacking systemic effects for the treatment of distal ulcerative colitis or proctitis. Scandinavian Journal of Gastroenterology 1992;27(1):9‐12. - PubMed
De Cassan 2012
-
- Cassan C, Fiorino G, Danese S. Second‐generation corticosteroids for the treatment of Crohn's disease and ulcerative colitis: more effective and less side effects?. Digestive Diseases 2012;30(4):368‐75. - PubMed
Dignass 2012
-
- Dignass A, Lindsay JO, Sturm A, Windsor A, Colombet LF, Allez M, et al. Second European evidence‐based consensus on the diagnosis and management of ulcerative colitis part 2: current management. Journal of Crohn's and Colitis 2012;6(10):991‐1030. - PubMed
Dilger 2004
-
- Dilger K, Schwab M, Fromm MF. Identification of budesonide and prednisone as substrates of the intestinal drug efflux pump P‐glycoprotein. Inflammatory Bowel Diseases 2004;10(5):578‐83. - PubMed
Eaden 2001
Ekbom 1990
-
- Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population‐based study. New England Journal of Medicine 1990;323(18):1228‐33. - PubMed
Feagan 2013
-
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2013;369(8):699‐710. - PubMed
Ford 2011
-
- Ford AC, Bernstein CN, Khan KJ, Abreu MT, Marshall JK, Takkey NJ, Moayyedi P. Glucocorticosteroid therapy and inflammatory bowel disease: a systematic review and meta‐analysis. American Journal of Gastroenterology 2011;106(4):590‐9. - PubMed
Gionchetti 2014
-
- Gionchetti P, Praticò C, Rizzello F, Calafiore A, Capozzi N, Campieri M, Calabrese C. The role of budesonide‐MMX in active ulcerative colitis. Expert Review of Gastroenterology and Hepatology 2014;8(3):215‐22. - PubMed
Griffiths 2004
-
- Griffiths AM. Specificities of inflammatory bowel disease in childhood. Best Practice and Research. Clinical Gastroenterology 2004;18(3):509‐23. - PubMed
Guyatt 2008
Hanauer 1993
-
- Hanauer S, Schwartz J, Robinson M, Roufail W, Arora S, Cello J, et al. Mesalamine capsules for the treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. American Journal of Gastroenterology 1993;88(8):1188‐97. - PubMed
Hanauer 2006
-
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflammatory Bowel Diseases 2006;12 Suppl 1:S3‐9. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoy 2015
-
- Hoy SM. Budesonide MMX(®): a review of its use in patients with mild to moderate ulcerative colitis. Drugs 2015;75(8):879‐86. - PubMed
Itzkowitz 2004
-
- Itzkowitz SH, Yio X. Colorectal cancer in inflammatory bowel disease: the role of inflammation. American Journal of Physiology. Gastrointestinal and Liver Physiology 2004;287(1):G7‐17. - PubMed
Jönsson 1995
-
- Jönsson G, Aström A, Andersson P. Budesonide is metabolized by cytochrome P450 3A (CYP3A) enzymes in human liver. Drug Metabolism and Disposition: The Biological Fate of Chemicals 1995;23(1):137‐42. - PubMed
Kirsner 2001
Kornbluth 2010
-
- Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. American Journal of Gastroenterology 2010;105(3):501‐23. - PubMed
Kozuch 2008
Kucharzik 2006
-
- Kucharzik T, Maaser C, Lügering A, Kagnoff M, Mayer L, Targan S, et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflammatory Bowel Diseases 2006;12(11):1068‐83. - PubMed
Kuenzig 2014
Lakatos 2006
-
- Lakatos L, Mester G, Erdelyi Z, David G, Pandur T, Balogh M, et al. Risk factors for ulcerative colitis‐associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population‐based study. Inflammatory Bowel Diseases 2006;12(3):205‐11. - PubMed
Lawson 2006
Lichtenstein 2006
-
- Lichtenstein GR, Abreu MT, Cohen R, Tremaine W, American Gastroenterological Association. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006;130(3):940‐87. - PubMed
Lichtenstein 2009
-
- Lichtenstein GR, Bengtsson B, Hapten‐White L, Rutgeerts P. Oral budesonide for maintenance of remission of Crohn's disease: a pooled safety analysis. Alimentary Pharmacology and Therapeutics 2009;29(6):643‐53. - PubMed
Lichtiger 1990
-
- Lichtiger S, Present DH. Preliminary report: cyclosporin in treatment of severe active ulcerative colitis. Lancet 1990;336(8706):16‐9. - PubMed
Lockhart‐Mummery 1960
Nos 2001
-
- Nos P, Hinojosa J. Gomollón F, Ponce J. Budesonide in inflammatory bowel disease: a meta‐analysis [Metaanálisis sobre la efectividad de la budesonida en la enfermedad inflamatoria intestinal]. Medicina Clínica 2001;116(2):47‐53. - PubMed
Powell‐Tuck 1978
-
- Powell‐Tuck J, Bown RL, Lennard‐Jones JE. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scandinavian Journal of Gastroenterology 1978;13(7):833‐7. - PubMed
Rachmilewitz 1989
Rezaie 2015
Riley 1991
Rutgeerts 2005
-
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2005;353(23):2462‐76. - PubMed
Rutter 2004
-
- Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004;126(2):451‐9. - PubMed
Sabate 1998
-
- Sabate JM, Chaussade S. Modalities of corticosteroid therapy in inflammatory bowel disease [Modalités de la corticothérapie au cours des maladies inflammatoires du tube digestif]. Semaine des Hôpitaux de Paris 1998;74:1079‐89.
Sands 2007
Saverymuttu 1986
-
- Saverymuttu SH, Camilleri M, Rees H, Lavender JP, Hodgson HJ, Chadwick VS. Indium 111‐granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111‐graunlocyte excretion. Gastroenterology 1986;90(5 Pt 1):1121‐8. - PubMed
Schroeder 1987
-
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. New England Journal of Medicine 1987;317(26):1625‐9. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Seo 1992
-
- Seo M, Okada M, Yao T, Ueki M, Arima A, Okumura M. An index of disease activity in patients with ulcerative colitis. American Journal of Gastroenterology 1992;87(8):971‐6. - PubMed
Seow 2009
-
- Seow CH, Benchimol EI, Steinhart AH, Griffiths AM, Otley AR. Budesonide for Crohn's disease. Expert Opinion on Drug Metabolism and Toxicology 2009;5(8):971‐9. - PubMed
Sutherland 1987
-
- Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N, Saibil F, et al. 5‐Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis and proctitis. Gastroenterology 1987;92(6):1894‐8. - PubMed
Thomsen 1998
-
- Thomsen OO, Cortot A, Jewell D, Wright JP, Winter T, Veloso FT, et al. A comparison of budesonide and mesalamine for active Crohn's disease. International Budesonide‐Mesalamine Study Group. New England Journal of Medicine 1998;339(6):370‐4. - PubMed
Timmer 2012
Travis 2006
-
- Travis SP. Review article: induction therapy for patients with active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2006;24(s1):10‐6. - PubMed
Travis 2012a
Truelove 1955
Turner 2007
-
- Turner D, Otley AR, Mack D, Hyams J, Bruijne J, Uusoue K, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133(2):423‐32. - PubMed
Van Limbergen 2008
-
- Limbergen J, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, et al. Definition of the phenotypic characteristics of childhood‐onset inflammatory bowel disease. Gastroenterology 2008;135(4):1114‐22. - PubMed
Walmsley 1998
Xavier 2007
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448(7152):427‐34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

