Cytotoxicity of PEGylated liposomes co-loaded with novel pro-apoptotic drug NCL-240 and the MEK inhibitor cobimetinib against colon carcinoma in vitro
- PMID: 26497930
- PMCID: PMC4688222
- DOI: 10.1016/j.jconrel.2015.10.037
Cytotoxicity of PEGylated liposomes co-loaded with novel pro-apoptotic drug NCL-240 and the MEK inhibitor cobimetinib against colon carcinoma in vitro
Abstract
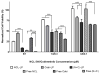
The overactivation of signaling pathways, such as the PI3K and MAPK, which are crucial to cell growth and survival, is a common feature in many cancer types. Though a number of advances have been made in the development of molecular agents targeting these pathways, their application as monotherapies has not significantly improved clinical outcome. A novel liposomal preparation was developed, co-loaded with NCL-240, a small-molecule inhibitor of the PI3K/mTOR pathway, along with cobimetinib, a MEK/ERK pathway inhibitor. This combination drug-loaded nanocarrier, (N+C)-LP, was able to significantly enhance the cytotoxicity of these drugs against colon carcinoma cells in vitro demonstrating a clear synergistic effect (combination index of 0.79). The (N+C)-LP was also able to induce cell cycle arrest of the cells, specifically in the G1 phase thereby preventing their progression to the S-phase, typical of the action of MEK inhibitors. Analyzing the apoptotic events, it was found that this effect on cell cycle regulation is followed by the induction of apoptosis. The quantified distribution of apoptotic events showed that the (N+C)-LP induced apoptosis significantly by over 3-4 fold (P<0.001) compared to other treatment groups. The co-loaded liposomal preparation was also targeted to the transferrin receptor of cancer cells by modifying the surface of the liposome with transferrin. FACS analysis showed that transferrin-mediated targeting enhanced the association of liposomes to HCT 116 cells by almost 5-fold. This could potentially allow for cancer cell-specific effects in vivo thereby minimizing any non-specific interactions of the liposomes with non-cancerous cells. Taken together, this study clearly shows that the combined inhibition of the PI3K and MEK pathways correlates with a significant anti-proliferative effect, due to cell-cycle regulation leading to the induction of apoptosis.
Keywords: Cobimetinib; Colon carcinoma; Liposomes; MEK; NCL-240; PI3K/AKT.
Copyright © 2015. Published by Elsevier B.V.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. - PubMed
-
- Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10(9):671–84. - PubMed
-
- Riehle RD, Cornea S, Degterev A. Lipid-mediated Protein Signaling. Springer; 2013. Role of phosphatidylinositol 3, 4, 5-trisphosphate in cell signaling; pp. 105–139. - PubMed
-
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773(8):1263–84. - PMC - PubMed
-
- Neuzillet C, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, Raymond E. MEK in cancer and cancer therapy. Pharmacol Ther. 2014;141(2):160–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

