Brucella abortus Cell Cycle and Infection Are Coordinated
- PMID: 26497941
- PMCID: PMC8800490
- DOI: 10.1016/j.tim.2015.09.007
Brucella abortus Cell Cycle and Infection Are Coordinated
Abstract
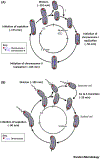
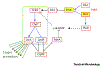
Brucellae are facultative intracellular pathogens. The recent development of methods and genetically engineered strains allowed the description of cell-cycle progression of Brucella abortus, including unipolar growth and the ordered initiation of chromosomal replication. B. abortus cell-cycle progression is coordinated with intracellular trafficking in the endosomal compartments. Bacteria are first blocked at the G1 stage, growth and chromosome replication being resumed shortly before reaching the intracellular proliferation compartment. The control mechanisms of cell cycle are similar to those reported for the bacterium Caulobacter crescentus, and they are crucial for survival in the host cell. The development of single-cell analyses could also be applied to other bacterial pathogens to investigate their cell-cycle progression during infection.
Keywords: Brucella; cell cycle; growth; intracellular infection; replication.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
G1-arrested newborn cells are the predominant infectious form of the pathogen Brucella abortus.Nat Commun. 2014 Jul 9;5:4366. doi: 10.1038/ncomms5366. Nat Commun. 2014. PMID: 25006695 Free PMC article.
-
A T4SS Effector Targets Host Cell Alpha-Enolase Contributing to Brucella abortus Intracellular Lifestyle.Front Cell Infect Microbiol. 2016 Nov 16;6:153. doi: 10.3389/fcimb.2016.00153. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27900285 Free PMC article.
-
B Lymphocytes provide an infection niche for intracellular bacterium Brucella abortus.J Infect Dis. 2012 Jul 1;206(1):91-8. doi: 10.1093/infdis/jis310. Epub 2012 May 4. J Infect Dis. 2012. PMID: 22561364 Free PMC article.
-
Brucella abortus DNA is a major bacterial agonist to activate the host innate immune system.Microbes Infect. 2014 Dec;16(12):979-84. doi: 10.1016/j.micinf.2014.08.010. Epub 2014 Aug 27. Microbes Infect. 2014. PMID: 25173577 Review.
-
Invasion and intracellular trafficking of Brucella abortus in nonphagocytic cells.Microbes Infect. 2000 Jun;2(7):829-35. doi: 10.1016/s1286-4579(00)90368-x. Microbes Infect. 2000. PMID: 10955964 Review.
Cited by
-
Brucella BtpB Manipulates Apoptosis and Autophagic Flux in RAW264.7 Cells.Int J Mol Sci. 2022 Nov 20;23(22):14439. doi: 10.3390/ijms232214439. Int J Mol Sci. 2022. PMID: 36430916 Free PMC article.
-
Brucellosis: Bacteriology, pathogenesis, epidemiology and role of the metallophores in virulence: a review.Front Cell Infect Microbiol. 2025 Jul 8;15:1621230. doi: 10.3389/fcimb.2025.1621230. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40697821 Free PMC article. Review.
-
Transposon Sequencing of Brucella abortus Uncovers Essential Genes for Growth In Vitro and Inside Macrophages.Infect Immun. 2018 Jul 23;86(8):e00312-18. doi: 10.1128/IAI.00312-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29844240 Free PMC article.
-
Uncovering the Hidden Credentials of Brucella Virulence.Microbiol Mol Biol Rev. 2021 Feb 10;85(1):e00021-19. doi: 10.1128/MMBR.00021-19. Print 2021 Feb 17. Microbiol Mol Biol Rev. 2021. PMID: 33568459 Free PMC article. Review.
-
The Brucella Cell Envelope.Annu Rev Microbiol. 2023 Sep 15;77:233-253. doi: 10.1146/annurev-micro-032521-013159. Epub 2023 Apr 27. Annu Rev Microbiol. 2023. PMID: 37104660 Free PMC article. Review.
References
-
- Moreno E and Moriyon I (2006) The genus Brucella. Prokaryotes 5, 315–456
-
- Archambaud C et al. (2010) Contrasting roles of macrophages and dendritic cells in controlling initial pulmonary Brucella infection. Eur. J. Immunol 40, 3458–3471 - PubMed
-
- Anderson TD et al. (1986) Pathogenesis of placentitis in the goat inoculated with Brucella abortus. II. Ultrastructural studies. Vet. Pathol 23, 227–239 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

